Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling
- PMID: 22733812
- PMCID: PMC3431702
- DOI: 10.1074/jbc.M112.383380
Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling
Abstract
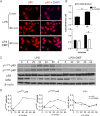
Dimethyl fumarate (DMF) is an effective novel treatment for multiple sclerosis in clinical trials. A reduction of IFN-γ-producing CD4(+) T cells is observed in DMF-treated patients and may contribute to its clinical efficacy. However, the cellular and molecular mechanisms behind this clinical observation are unclear. In this study, we investigated the effects of DMF on dendritic cell (DC) maturation and subsequent DC-mediated T cell responses. We show that DMF inhibits DC maturation by reducing inflammatory cytokine production (IL-12 and IL-6) and the expression of MHC class II, CD80, and CD86. Importantly, this immature DC phenotype generated fewer activated T cells that were characterized by decreased IFN-γ and IL-17 production. Further molecular studies demonstrated that DMF impaired nuclear factor κB (NF-κB) signaling via reduced p65 nuclear translocalization and phosphorylation. NF-κB signaling was further decreased by DMF-mediated suppression of extracellular signal-regulated kinase 1 and 2 (ERK1/2) and its downstream kinase mitogen stress-activated kinase 1 (MSK1). MSK1 suppression resulted in decreased p65 phosphorylation at serine 276 and reduced histone phosphorylation at serine 10. As a consequence, DMF appears to reduce p65 transcriptional activity both directly and indirectly by promoting a silent chromatin environment. Finally, treatment of DCs with the MSK1 inhibitor H89 partially mimicked the effects of DMF on the DC signaling pathway and impaired DC maturation. Taken together, these studies indicate that by suppression of both NF-κB and ERK1/2-MSK1 signaling, DMF inhibits maturation of DCs and subsequently Th1 and Th17 cell differentiation.
Figures





Similar articles
-
Dimethylfumarate inhibits NF-{kappa}B function at multiple levels to limit airway smooth muscle cell cytokine secretion.Am J Physiol Lung Cell Mol Physiol. 2009 Aug;297(2):L326-39. doi: 10.1152/ajplung.90624.2008. Epub 2009 May 22. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19465513
-
NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway.J Biol Chem. 2008 Jun 13;283(24):16391-9. doi: 10.1074/jbc.M800945200. Epub 2008 Apr 18. J Biol Chem. 2008. PMID: 18424438 Free PMC article.
-
Dimethylfumarate specifically inhibits the mitogen and stress-activated kinases 1 and 2 (MSK1/2): possible role for its anti-psoriatic effect.J Invest Dermatol. 2007 Sep;127(9):2129-37. doi: 10.1038/sj.jid.5700859. Epub 2007 May 10. J Invest Dermatol. 2007. PMID: 17495961
-
Dimethyl fumarate: A review of preclinical efficacy in models of neurodegenerative diseases.Eur J Pharmacol. 2022 Jul 5;926:175025. doi: 10.1016/j.ejphar.2022.175025. Epub 2022 May 13. Eur J Pharmacol. 2022. PMID: 35569547 Review.
-
Dimethyl fumarate modulation of immune and antioxidant responses: application to HIV therapy.Crit Rev Immunol. 2013;33(4):307-59. doi: 10.1615/critrevimmunol.2013007247. Crit Rev Immunol. 2013. PMID: 23971529 Free PMC article. Review.
Cited by
-
Anti-inflammatory dimethylfumarate: a potential new therapy for asthma?Mediators Inflamm. 2013;2013:875403. doi: 10.1155/2013/875403. Epub 2013 Mar 27. Mediators Inflamm. 2013. PMID: 23606796 Free PMC article. Review.
-
Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer.Pharmacol Ther. 2019 Feb;194:59-72. doi: 10.1016/j.pharmthera.2018.09.004. Epub 2018 Sep 19. Pharmacol Ther. 2019. PMID: 30240635 Free PMC article. Review.
-
Dimethyl fumarate impairs differentiated B cells and fosters central nervous system integrity in treatment of multiple sclerosis.Brain Pathol. 2019 Sep;29(5):640-657. doi: 10.1111/bpa.12711. Epub 2019 Mar 5. Brain Pathol. 2019. PMID: 30706542 Free PMC article.
-
Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia.Front Immunol. 2017 Nov 10;8:1520. doi: 10.3389/fimmu.2017.01520. eCollection 2017. Front Immunol. 2017. PMID: 29176977 Free PMC article. Review.
-
Dimethyl fumarate as a first- vs second-line therapy in MS: Focus on B cells.Neurol Neuroimmunol Neuroinflamm. 2018 Oct 16;5(6):e508. doi: 10.1212/NXI.0000000000000508. eCollection 2018 Nov. Neurol Neuroimmunol Neuroinflamm. 2018. PMID: 30345334 Free PMC article.
References
-
- Frohman E. M., Racke M. K., Raine C. S. (2006) Multiple sclerosis–the plaque and its pathogenesis. N. Engl. J. Med. 354, 942–955 - PubMed
-
- Steinman R. M., Banchereau J. (2007) Taking dendritic cells into medicine. Nature 449, 419–426 - PubMed
-
- Karni A., Abraham M., Monsonego A., Cai G., Freeman G. J., Hafler D., Khoury S. J., Weiner H. L. (2006) Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 177, 4196–4202 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

