Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research
- PMID: 22733976
- PMCID: PMC3555312
- DOI: 10.1136/amiajnl-2011-000681
Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research
Abstract
Objective: To review the methods and dimensions of data quality assessment in the context of electronic health record (EHR) data reuse for research.
Materials and methods: A review of the clinical research literature discussing data quality assessment methodology for EHR data was performed. Using an iterative process, the aspects of data quality being measured were abstracted and categorized, as well as the methods of assessment used.
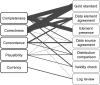
Results: Five dimensions of data quality were identified, which are completeness, correctness, concordance, plausibility, and currency, and seven broad categories of data quality assessment methods: comparison with gold standards, data element agreement, data source agreement, distribution comparison, validity checks, log review, and element presence.
Discussion: Examination of the methods by which clinical researchers have investigated the quality and suitability of EHR data for research shows that there are fundamental features of data quality, which may be difficult to measure, as well as proxy dimensions. Researchers interested in the reuse of EHR data for clinical research are recommended to consider the adoption of a consistent taxonomy of EHR data quality, to remain aware of the task-dependence of data quality, to integrate work on data quality assessment from other fields, and to adopt systematic, empirically driven, statistically based methods of data quality assessment.
Conclusion: There is currently little consistency or potential generalizability in the methods used to assess EHR data quality. If the reuse of EHR data for clinical research is to become accepted, researchers should adopt validated, systematic methods of EHR data quality assessment.
Conflict of interest statement
Figures
References
-
- Hersh WR. Adding value to the electronic health record through secondary use of data for quality assurance, research, and surveillance. Am J Manag Care 2007;13:277–8 - PubMed
-
- National Center for Research Resources (US) Widening the Use of Electronic Health Record Data for Research. 2009. http://videocast.nih.gov/summary.asp?live=8062 (accessed 9 Oct 2011).
-
- Weiner MG, Embi PJ. Toward reuse of clinical data for research and quality improvement: the end of the beginning? Ann Intern Med 2009;151:359–60 - PubMed
-
- Burnum JF. The misinformation era: the fall of the medical record. Ann Intern Med 1989;110:482–4 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

