Coordinate regulation of TPL-2 and NF-κB signaling in macrophages by NF-κB1 p105
- PMID: 22733995
- PMCID: PMC3422004
- DOI: 10.1128/MCB.00564-12
Coordinate regulation of TPL-2 and NF-κB signaling in macrophages by NF-κB1 p105
Abstract
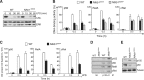
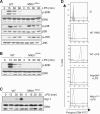
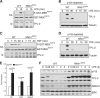
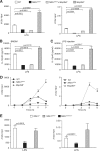
The role of IκB kinase (IKK)-induced proteolysis of NF-κB1 p105 in innate immune signaling was investigated using macrophages from Nfkb1(SSAA/SSAA) mice, in which the IKK target serines on p105 are mutated to alanines. We found that the IKK/p105 signaling pathway was essential for TPL-2 kinase activation of extracellular signal-regulated kinase (ERK) mitogen-activate protein (MAP) kinase and modulated the activation of NF-κB. The Nfkb1(SSAA) mutation prevented the agonist-induced release of TPL-2 from its inhibitor p105, which blocked activation of ERK by lipopolysaccharide (LPS), tumor necrosis factor (TNF), CpG, tripalmitoyl-Cys-Ser-Lys (Pam(3)CSK), poly(I · C), flagellin, and R848. The Nfkb1(SSAA) mutation also prevented LPS-induced processing of p105 to p50 and reduced p50 levels, in addition to decreasing the nuclear translocation of RelA and cRel. Reduced p50 in Nfkb1(SSAA/SSAA) macrophages significantly decreased LPS induction of the IκBζ-regulated Il6 and Csf2 genes. LPS upregulation of Il12a and Il12b mRNAs was also impaired although specific blockade of TPL-2 signaling increased expression of these genes at late time points. Activation of TPL-2/ERK signaling by IKK-induced p105 proteolysis, therefore, induced a negative feedback loop to downregulate NF-κB-dependent expression of the proinflammatory cytokine interleukin-12 (IL-12). Unexpectedly, TPL-2 promoted soluble TNF production independently of IKK-induced p105 phosphorylation and its ability to activate ERK, which has important implications for the development of anti-inflammatory drugs targeting TPL-2.
Figures




References
-
- Aoki M, et al. 1993. The human cot proto-oncogene encodes two protein serine/threonine kinases with different transforming activities by alternative initiation of translation. J. Biol. Chem. 268: 22723– 22732 - PubMed
-
- Babu GR, et al. 2006. Phosphorylation of NF-κB1/p105 by oncoprotein kinase Tpl2: implications for a novel mechanism of Tpl2 regulation. Biochim. Biophys. Acta 1763: 174– 181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
