The impact of the unfolded protein response on human disease
- PMID: 22733998
- PMCID: PMC3384412
- DOI: 10.1083/jcb.201110131
The impact of the unfolded protein response on human disease
Abstract
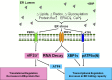
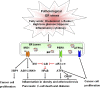
A central function of the endoplasmic reticulum (ER) is to coordinate protein biosynthetic and secretory activities in the cell. Alterations in ER homeostasis cause accumulation of misfolded/unfolded proteins in the ER. To maintain ER homeostasis, eukaryotic cells have evolved the unfolded protein response (UPR), an essential adaptive intracellular signaling pathway that responds to metabolic, oxidative stress, and inflammatory response pathways. The UPR has been implicated in a variety of diseases including metabolic disease, neurodegenerative disease, inflammatory disease, and cancer. Signaling components of the UPR are emerging as potential targets for intervention and treatment of human disease.
Figures
References
-
- Auf G., Jabouille A., Guérit S., Pineau R., Delugin M., Bouchecareilh M., Magnin N., Favereaux A., Maitre M., Gaiser T., et al. 2010. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc. Natl. Acad. Sci. USA. 107:15553–15558 10.1073/pnas.0914072107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

