Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone
- PMID: 22734013
- PMCID: PMC3403730
- DOI: 10.1093/annonc/mds178
Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone
Abstract
Background: Malignant ascites (MA) is associated with poor prognosis and limited palliative therapeutic options. Therefore, quality of life (QoL) assessment is of particular importance to demonstrate new treatment value. Following the demonstration of the superiority of catumaxomab and paracentesis over paracentesis on puncture-free survival, this analysis aimed at comparing deterioration in QoL between both the treatment options.
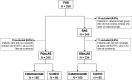
Patients and methods: In a randomised, multicentre, phase II/III study of patients with MA due to epithelial cell adhesion molecule (EpCAM) positive cancer, the QoL was evaluated using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 items (EORTC QLQ-C30) questionnaire at screening, 1, 3 and 7 months after treatment and in the case of re-puncture on the day of paracentesis. Time to first deterioration in QoL was defined as a decrease in the QoL score of at least five points and compared between the catumaxomab (n=160) and control (n=85) groups using the log-rank test and Cox proportional hazards models adjusted for baseline score, country and primary tumour type.
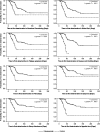
Results: Deterioration in QoL scores appeared more rapidly in the control than in the catumaxomab group (median 19-26 days versus 47-49 days). The difference in time to deterioration in QoL between the groups was statistically significant for all scores (P<0.01). The hazard ratios ranged from 0.08 to 0.24 (P<0.01).
Conclusions: Treatment with catumaxomab delayed deterioration in QoL in patients with MA. Compared with paracentesis alone, catumaxomab enabled patients to benefit from better QoL for a prolonged survival period.
Trial registration: ClinicalTrials.gov NCT00836654.
Figures

Similar articles
-
Catumaxomab: in malignant ascites.Drugs. 2012 Jul 9;72(10):1399-410. doi: 10.2165/11209040-000000000-00000. Drugs. 2012. PMID: 22676343 Review.
-
The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial.Int J Cancer. 2010 Nov 1;127(9):2209-21. doi: 10.1002/ijc.25423. Int J Cancer. 2010. PMID: 20473913 Free PMC article. Clinical Trial.
-
The role of relative lymphocyte count as a biomarker for the effect of catumaxomab on survival in malignant ascites patients: results from a phase II/III study.Clin Cancer Res. 2014 Jun 15;20(12):3348-57. doi: 10.1158/1078-0432.CCR-13-2351. Epub 2014 Apr 8. Clin Cancer Res. 2014. PMID: 24714773 Clinical Trial.
-
Catumaxomab--trifunctional anti-EpCAM antibody used to treat malignant ascites.Expert Opin Biol Ther. 2010 Aug;10(8):1259-69. doi: 10.1517/14712598.2010.504706. Expert Opin Biol Ther. 2010. PMID: 20624115 Review.
-
Detection of soluble EpCAM (sEpCAM) in malignant ascites predicts poor overall survival in patients treated with catumaxomab.Oncotarget. 2015 Sep 22;6(28):25017-23. doi: 10.18632/oncotarget.4496. Oncotarget. 2015. PMID: 26296970 Free PMC article.
Cited by
-
Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization?Qual Life Res. 2015 Jan;24(1):5-18. doi: 10.1007/s11136-013-0583-6. Epub 2013 Nov 26. Qual Life Res. 2015. PMID: 24277234 Free PMC article.
-
An EpCAM/CD3 bispecific antibody efficiently eliminates hepatocellular carcinoma cells with limited galectin-1 expression.Cancer Immunol Immunother. 2014 Feb;63(2):121-32. doi: 10.1007/s00262-013-1497-4. Epub 2013 Nov 1. Cancer Immunol Immunother. 2014. PMID: 24177984 Free PMC article.
-
Proceedings From the First International Workshop at Sidra Medicine: "Engineered Immune Cells in Cancer Immunotherapy (EICCI): From Discovery to Off-the-Shelf Development", 15th-16th February 2019, Doha, Qatar.Front Immunol. 2021 Jan 14;11:589381. doi: 10.3389/fimmu.2020.589381. eCollection 2020. Front Immunol. 2021. PMID: 33584653 Free PMC article.
-
Immunotherapy for targeting cancer stem cells in hepatocellular carcinoma.Theranostics. 2021 Jan 19;11(7):3489-3501. doi: 10.7150/thno.54648. eCollection 2021. Theranostics. 2021. PMID: 33537099 Free PMC article. Review.
-
Tunneled Peritoneal Catheter Placement in Palliation of Malignant Ascites: A Study with Two Different Types of Catheters.Biomed Res Int. 2019 May 30;2019:4132396. doi: 10.1155/2019/4132396. eCollection 2019. Biomed Res Int. 2019. PMID: 31275969 Free PMC article.
References
-
- Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–949. - PubMed
-
- Feld R. Endpoints in cancer clinical trials: is there a need for measuring quality of life? Support Care Cancer. 1995;3:23–27. - PubMed
-
- Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458–467. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

