Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group
- PMID: 22734022
- PMCID: PMC3402886
- DOI: 10.1200/JCO.2011.40.8724
Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group
Abstract
Purpose: Children's Oncology Group study AALL00P2 was designed to assess the feasibility and safety of adding nelarabine to a BFM 86-based chemotherapy regimen in children with newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL).
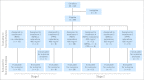
Patients and methods: In stage one of the study, eight patients with a slow early response (SER) by prednisone poor response (PPR; ≥ 1,000 peripheral blood blasts on day 8 of prednisone prephase) received chemotherapy plus six courses of nelarabine 400 mg/m(2) once per day; four patients with SER by high minimal residual disease (MRD; ≥ 1% at day 36 of induction) received chemotherapy plus five courses of nelarabine; 16 patients with a rapid early response (RER) received chemotherapy without nelarabine. In stage two, all patients received six 5-day courses of nelarabine at 650 mg/m(2) once per day (10 SER patients [one by MRD, nine by PPR]) or 400 mg/m(2) once per day (38 RER patients; 12 SER patients [three by MRD, nine by PPR]).
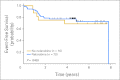
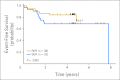
Results: The only significant difference in toxicities was decreased neutropenic infections in patients treated with nelarabine (42% with v 81% without nelarabine). Five-year event-free survival (EFS) rates were 73% for 11 stage one SER patients and 67% for 22 stage two SER patients treated with nelarabine versus 69% for 16 stage one RER patients treated without nelarabine and 74% for 38 stage two RER patients treated with nelarabine. Five-year EFS for all patients receiving nelarabine (n = 70) was 73% versus 69% for those treated without nelarabine (n = 16).
Conclusion: Addition of nelarabine to a BFM 86-based chemotherapy regimen was well tolerated and produced encouraging results in pediatric patients with T-ALL, particularly those with a SER, who have historically fared poorly.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Reiter A, Schrappe M, Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients: Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84:3122–3133. - PubMed
-
- Uckun FM, Reaman G, Steinherez PG, et al. Improved clinic outcome for children with T-lineage acute lymphoblastic leukemia after contemporary chemotherapy: A Children's Cancer Group study. Leuk Lymphoma. 1996;24:57–70. - PubMed
-
- Steinherz PG, Gaynon PS, Breneman JC, et al. Treatment of patients with acute lymphoblastic leukemia with bulky extramedullary disease and T-cell phenotype or other poor prognostic features. Cancer. 1998;82:600–612. - PubMed
-
- Laver J, Amylon M, Desai S, et al. Randomized trial of r-metHu granulocyte colony-stimulating factor in an intensive treatment for T-cell leukemia and advance-stage lymphoblastic lymphoma of childhood: A Pediatric Oncology Group pilot study. J Clin Oncol. 1998;16:522–526. - PubMed
-
- Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advance stage lymphoblastic lymphoma: A Pediatric Oncology Group study. Leukemia. 1999;13:335–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

