Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening
- PMID: 22734046
- PMCID: PMC3405507
- DOI: 10.1084/jem.20120033
Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening
Abstract
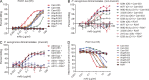
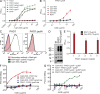
Pseudomonas aeruginosa is a leading cause of hospital-associated infections in the seriously ill, and the primary agent of chronic lung infections in cystic fibrosis patients. A major obstacle to effective control of P. aeruginosa infections is its intrinsic resistance to most antibiotic classes, which results from chromosomally encoded drug-efflux systems and multiple acquired resistance mechanisms selected by years of aggressive antibiotic therapy. These factors demand new strategies and drugs to prevent and treat P. aeruginosa infections. Herein, we describe a monoclonal antibody (mAb) selection strategy on whole P. aeruginosa cells using single-chain variable fragment phage libraries derived from healthy individuals and patients convalescing from P. aeruginosa infections. This approach enabled identification of mAbs that bind three distinct epitopes on the product of the Psl. This exopolysaccharide is important for P. aeruginosa attachment to mammalian cells, and for the formation and maintenance of biofilms produced by nonmucoid and mucoid P. aeruginosa isolates. Functional screens revealed that mAbs to one epitope exhibit superior activity in opsonophagocytic killing and cell attachment assays, and confer significant protection in multiple animal models. Our results indicate that Psl is an accessible serotype-independent surface feature and promising novel protective antigen for preventing P. aeruginosa infections. Furthermore, our mAb discovery strategy holds promise for application to other bacterial pathogens.
Figures






References
-
- Byrd M.S., Sadovskaya I., Vinogradov E., Lu H., Sprinkle A.B., Richardson S.H., Ma L., Ralston B., Parsek M.R., Anderson E.M., et al. 2009. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 73:622–638 10.1111/j.1365-2958.2009.06795.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

