Identification of a genetic locus controlling bacteria-driven colitis and associated cancer through effects on innate inflammation
- PMID: 22734048
- PMCID: PMC3405508
- DOI: 10.1084/jem.20120239
Identification of a genetic locus controlling bacteria-driven colitis and associated cancer through effects on innate inflammation
Abstract
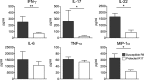
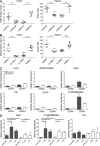
Chronic inflammation of the intestine has been associated with an elevated risk of developing colorectal cancer. Recent association studies have highlighted the role of genetic predisposition in the etiology of colitis and started to unravel its complexity. However, the genetic factors influencing the progression from colon inflammation to tumorigenesis are not known. We report the identification of a genetic interval Hiccs that regulates Helicobacter hepaticus-induced colitis and associated cancer susceptibility in a 129.RAG(-/-) mouse model. The 1.7-Mb congenic interval on chromosome 3, containing eight genes and five microRNAs, renders susceptible mice resistant to colitis and reduces tumor incidence and multiplicity. Bone marrow chimera experiments showed that resistance is conferred by the hematopoietic compartment. Moreover, the Hiccs locus controls the induction of the innate inflammatory response by regulating cytokine expression and granulocyte recruitment by Thy1(+) innate lymphoid cells. Using a tumor-promoting model combining chronic Helicobacter hepaticus infection and the carcinogen azoxymethane, we found that Hiccs also regulates the frequency of colitis-associated neoplasia. Our study highlights the importance of innate immune cells and their genetic configuration in driving progression from inflammation toward cancer and opens the door for analysis of these pathways in human inflammatory disorders and associated cancers.
Figures






References
-
- Anderson C.A., Boucher G., Lees C.W., Franke A., D’Amato M., Taylor K.D., Lee J.C., Goyette P., Imielinski M., Latiano A., et al. 2011. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43:246–252 10.1038/ng.764 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

