The complement system of the goat: haemolytic assays and isolation of major proteins
- PMID: 22734447
- PMCID: PMC3413586
- DOI: 10.1186/1746-6148-8-91
The complement system of the goat: haemolytic assays and isolation of major proteins
Abstract
Background: The aim of the present study was to develop a haemolytic assay for the study of the complement system in dairy goats (Capra aegagrus hircus) and to characterize the major goat complement system proteins.
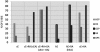
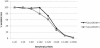
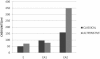
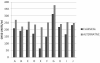
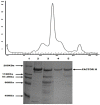
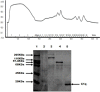
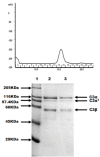
Results: The commonly used sheep erythrocyte sensitized with rabbit antibodies were not sensitive to lysis by goat serum, but the combination of human red blood cells (RBC) plus rabbit antibodies was the best option found for goat complement assay. A buffer based on HEPES instead of the classical veronal (barbitone) was developed. Three proteins were isolated: factor H, C1q and C3 and these were compared with the corresponding human proteins. A novel affinity chromatography technique was developed for isolation of factor H.
Conclusions: Human RBC plus rabbit antibodies were a suitable option for haemolytic assays. The isolated proteins are similar to the human counterparts.
Figures
References
-
- Mayilyan KR, Kang YH, Dodds AW, Sim RB. In: Innate Immunity of Plants, Animals and Humans. Heine H, editor. Berlín: Springer-Verlag Berlin Heidelberg; 2008. The complement system in innate immunity; pp. 219–236. 21.
-
- Seelen MA, Roos A, Wieslander J, Mollnes TE, Sjoholm AG, Wurzner R, Loos M, Tedesco F, Sim RB, Garred P, Alexopoulos E, Turner MW, Daha MR. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187–198. doi: 10.1016/j.jim.2004.11.016. - DOI - PubMed
-
- Matsushita M, Endo Y, Fujita T. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–2284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

