Molecular genetic studies and delineation of the oculocutaneous albinism phenotype in the Pakistani population
- PMID: 22734612
- PMCID: PMC3537634
- DOI: 10.1186/1750-1172-7-44
Molecular genetic studies and delineation of the oculocutaneous albinism phenotype in the Pakistani population
Abstract
Background: Oculocutaneous albinism (OCA) is caused by a group of genetically heterogeneous inherited defects that result in the loss of pigmentation in the eyes, skin and hair. Mutations in the TYR, OCA2, TYRP1 and SLC45A2 genes have been shown to cause isolated OCA. No comprehensive analysis has been conducted to study the spectrum of OCA alleles prevailing in Pakistani albino populations.
Methods: We enrolled 40 large Pakistani families and screened them for OCA genes and a candidate gene, SLC24A5. Protein function effects were evaluated using in silico prediction algorithms and ex vivo studies in human melanocytes. The effects of splice-site mutations were determined using an exon-trapping assay.
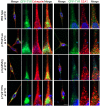
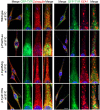
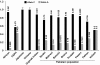
Results: Screening of the TYR gene revealed four known (p.Arg299His, p.Pro406Leu, p.Gly419Arg, p.Arg278*) and three novel mutations (p.Pro21Leu, p.Cys35Arg, p.Tyr411His) in ten families. Ex vivo studies revealed the retention of an EGFP-tagged mutant (p.Pro21Leu, p.Cys35Arg or p.Tyr411His) tyrosinase in the endoplasmic reticulum (ER) at 37°C, but a significant fraction of p.Cys35Arg and p.Tyr411His left the ER in cells grown at a permissive temperature (31°C). Three novel (p.Asp486Tyr, p.Leu527Arg, c.1045-15 T > G) and two known mutations (p.Pro743Leu, p.Ala787Thr) of OCA2 were found in fourteen families. Exon-trapping assays with a construct containing a novel c.1045-15 T > G mutation revealed an error in splicing. No mutation in TYRP1, SLC45A2, and SLC24A5 was found in the remaining 16 families. Clinical evaluation of the families segregating either TYR or OCA2 mutations showed nystagmus, photophobia, and loss of pigmentation in the skin or hair follicles. Most of the affected individuals had grayish-blue colored eyes.
Conclusions: Our results show that ten and fourteen families harbored mutations in the TYR and OCA2 genes, respectively. Our findings, along with the results of previous studies, indicate that the p.Cys35Arg, p.Arg278* and p.Gly419Arg alleles of TYR and the p.Asp486Tyr and c.1045-15 T > G alleles of OCA2 are the most common causes of OCA in Pakistani families. To the best of our knowledge, this study represents the first documentation of OCA2 alleles in the Pakistani population. A significant proportion of our cohort did not have mutations in known OCA genes. Overall, our study contributes to the development of genetic testing protocols and genetic counseling for OCA in Pakistani families.
Figures





References
-
- Seven M, Yosunkaya E, Yilmaz SB, Karaca E, Guven G, Yuksel A. A new syndrome presenting with dysmorphic facies, oculocutaneous albinism, glaucoma, cryptorchidism and mental retardation. Genet Couns. 2011;22:25–34. - PubMed
-
- Tripathi RK, Hearing VJ, Urabe K, Aroca P, Spritz RA. Mutational mapping of the catalytic activities of human tyrosinase. J Biol Chem. 1992;267:23707–23712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

