Development of peptide-based reversing agents for p-glycoprotein-mediated resistance to carfilzomib
- PMID: 22734651
- PMCID: PMC3473138
- DOI: 10.1021/mp300044b
Development of peptide-based reversing agents for p-glycoprotein-mediated resistance to carfilzomib
Abstract
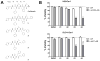
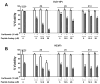
Carfilzomib is a novel class of peptidyl epoxyketone proteasome inhibitor and has demonstrated promising activity in multiple clinical trials to treat patients with multiple myeloma and other types of cancers. Here, we investigated molecular mechanisms underlying acquired resistance to carfilzomib and a potential strategy to restore cellular sensitivity to carfilzomib. H23 and DLD-1 cells (human lung and colon adenocarcinoma cell lines) with acquired resistance to carfilzomib displayed marked cross-resistance to YU-101, a closely related proteasome inhibitor, and paclitaxel, a known substrate of Pgp. However, carfilzomib-resistant cells remained sensitive to bortezomib, a clinically used dipeptide with boronic acid pharmacophore. In accordance with these observations, carfilzomib-resistant H23 and DLD-1 cells showed marked upregulation of P-glycoprotein (Pgp) as compared to their parental controls, and coincubation with verapamil, a Pgp inhibitor, led to an almost complete restoration of cellular sensitivity to carfilzomib. These results indicate that Pgp upregulation plays a major role in the development of carfilzomib resistance in these cell lines. In developing a potential strategy to overcome carfilzomib resistance, we as a proof of concept prepared a small library of peptide analogues derived from the peptide backbone of carfilzomib and screened these molecules for their activity to restore carfilzomib sensitivity when cotreated with carfilzomib. We found that compounds as small as dipeptides are sufficient in restoring carfilzomib sensitivity. Taken together, we found that Pgp upregulation plays a major role in the development of resistance to carfilzomib in lung and colon adenocarcinoma cell lines and that small peptide analogues lacking the pharmacophore can be used as agents to reverse acquired carfilzomib resistance. Our findings may provide important information in developing a potential strategy to overcome drug resistance.
Figures

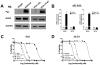
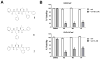
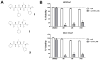
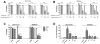
References
-
- Marques AJ, Palanimurugan R, Matias AC, Ramos PC, Dohmen RJ. Catalytic mechanism and assembly of the proteasome. Chem Rev. 2009;109:1509–1536. - PubMed
-
- Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. - PubMed
-
- Kuhn DJ, Orlowski RZ, Bjorklund CC. Second Generation Proteasome Inhibitors: Carfilzomib and Immunoproteasome-Specific Inhibitors (IPSIs) Curr Cancer Drug Targets. 2011;11:285–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

