IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments
- PMID: 22734718
- PMCID: PMC3423045
- DOI: 10.1186/1742-2094-9-144
IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments
Abstract
Background: In immune-mediated diseases of the central nervous system, astrocytes exposed to interferon-γ (IFN-γ) can express major histocompatibility complex (MHC) class II molecules and antigens on their surface. MHC class II molecules are thought to be delivered to the cell surface by membrane-bound vesicles. However, the characteristics and dynamics of this vesicular traffic are unclear, particularly in reactive astrocytes, which overexpress intermediate filament (IF) proteins that may affect trafficking. The aim of this study was to determine the mobility of MHC class II vesicles in wild-type (WT) astrocytes and in astrocytes devoid of IFs.
Methods: The identity of MHC class II compartments in WT and IF-deficient astrocytes 48 h after IFN-γ activation was determined immunocytochemically by using confocal microscopy. Time-lapse confocal imaging and Alexa Fluor546-dextran labeling of late endosomes/lysosomes in IFN-γ treated cells was used to characterize the motion of MHC class II vesicles. The mobility of vesicles was analyzed using ParticleTR software.
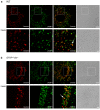
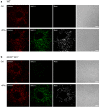
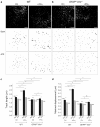
Results: Confocal imaging of primary cultures of WT and IF-deficient astrocytes revealed IFN-γ induced MHC class II expression in late endosomes/lysosomes, which were specifically labeled with Alexa Fluor546-conjugated dextran. Live imaging revealed faster movement of dextran-positive vesicles in IFN-γ-treated than in untreated astrocytes. Vesicle mobility was lower in IFN-γ-treated IF-deficient astrocytes than in WT astrocytes. Thus, the IFN-γ-induced increase in the mobility of MHC class II compartments is IF-dependent.
Conclusions: Since reactivity of astrocytes is a hallmark of many CNS pathologies, it is likely that the up-regulation of IFs under such conditions allows a faster and therefore a more efficient delivery of MHC class II molecules to the cell surface. In vivo, such regulatory mechanisms may enable antigen-presenting reactive astrocytes to respond rapidly and in a controlled manner to CNS inflammation.
Figures



Similar articles
-
Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes.Glia. 2010 Aug;58(10):1208-19. doi: 10.1002/glia.21000. Glia. 2010. PMID: 20544856
-
Fingolimod Suppresses the Proinflammatory Status of Interferon-γ-Activated Cultured Rat Astrocytes.Mol Neurobiol. 2019 Sep;56(9):5971-5986. doi: 10.1007/s12035-019-1481-x. Epub 2019 Jan 30. Mol Neurobiol. 2019. PMID: 30701416
-
Exocytosis of large-diameter lysosomes mediates interferon γ-induced relocation of MHC class II molecules toward the surface of astrocytes.Cell Mol Life Sci. 2020 Aug;77(16):3245-3264. doi: 10.1007/s00018-019-03350-8. Epub 2019 Oct 30. Cell Mol Life Sci. 2020. PMID: 31667557 Free PMC article.
-
Interferon-gamma mediates antigen trafficking to MHC class II-positive late endosomes of enterocytes.Eur J Immunol. 2005 Mar;35(3):831-42. doi: 10.1002/eji.200425286. Eur J Immunol. 2005. PMID: 15688349
-
Astrocytic vesicle mobility in health and disease.Int J Mol Sci. 2013 May 27;14(6):11238-58. doi: 10.3390/ijms140611238. Int J Mol Sci. 2013. PMID: 23712361 Free PMC article. Review.
Cited by
-
Syk and Hrs Regulate TLR3-Mediated Antiviral Response in Murine Astrocytes.Oxid Med Cell Longev. 2019 Apr 4;2019:6927380. doi: 10.1155/2019/6927380. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31089414 Free PMC article.
-
Differential Cytokine-Induced Responses of Polarized Microglia.Brain Sci. 2021 Nov 10;11(11):1482. doi: 10.3390/brainsci11111482. Brain Sci. 2021. PMID: 34827481 Free PMC article.
-
Astroglia in Sepsis Associated Encephalopathy.Neurochem Res. 2020 Jan;45(1):83-99. doi: 10.1007/s11064-019-02743-2. Epub 2019 Feb 18. Neurochem Res. 2020. PMID: 30778837 Free PMC article. Review.
-
Olfactory Ensheathing Cells Inhibit Gliosis in Retinal Degeneration by Downregulation of the Müller Cell Notch Signaling Pathway.Cell Transplant. 2017 Jun 9;26(6):967-982. doi: 10.3727/096368917X694994. Epub 2017 Feb 9. Cell Transplant. 2017. PMID: 28185609 Free PMC article.
-
Slow degradation in phagocytic astrocytes can be enhanced by lysosomal acidification.Glia. 2015 Nov;63(11):1997-2009. doi: 10.1002/glia.22873. Epub 2015 Jun 12. Glia. 2015. PMID: 26095880 Free PMC article.
References
-
- Shrikant P, Benveniste EN. The central nervous system as an immunocompetent organ: role of glial cells in antigen presentation. J Immunol. 1996;157:1819–1822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

