Microscopic magnetic stimulation of neural tissue
- PMID: 22735449
- PMCID: PMC3621430
- DOI: 10.1038/ncomms1914
Microscopic magnetic stimulation of neural tissue
Abstract
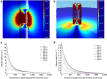
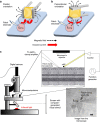
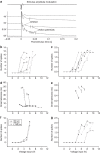
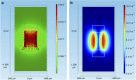
Electrical stimulation is currently used to treat a wide range of cardiovascular, sensory and neurological diseases. Despite its success, there are significant limitations to its application, including incompatibility with magnetic resonance imaging, limited control of electric fields and decreased performance associated with tissue inflammation. Magnetic stimulation overcomes these limitations but existing devices (that is, transcranial magnetic stimulation) are large, reducing their translation to chronic applications. In addition, existing devices are not effective for deeper, sub-cortical targets. Here we demonstrate that sub-millimeter coils can activate neuronal tissue. Interestingly, the results of both modelling and physiological experiments suggest that different spatial orientations of the coils relative to the neuronal tissue can be used to generate specific neural responses. These results raise the possibility that micro-magnetic stimulation coils, small enough to be implanted within the brain parenchyma, may prove to be an effective alternative to existing stimulation devices.
Figures


References
-
- Wichmann T. & Delong M. R. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52, 197–204 (2006). - PubMed
-
- Montgomery E. B. Jr & Gale J. T. Mechanisms of action of deep brain stimulation(DBS). Neurosci. Biobehav. Rev. 32, 388–407 (2008). - PubMed
-
- Wilson B. S. & Dorman M. F. Cochlear implants: current designs and future possibilities. J. Rehabil. Res. Dev. 45, 695–730 (2008). - PubMed
-
- Laferrier J. Z. & Gailey R. Advances in lower-limb prosthetic technology. Phys. Med. Rehabil. Clin. N. Am. 21, 87–110 (2010). - PubMed
-
- Loewenstein J. I., Montezuma S. R. & Rizzo J. F. 3rd Outer retinal degeneration: an electronic retinal prosthesis as a treatment strategy. Arch. Ophthalmol. 122, 587–596 (2004). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

