ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer
- PMID: 22735453
- PMCID: PMC3871175
- DOI: 10.1038/ncomms1927
ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer
Abstract
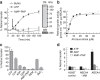
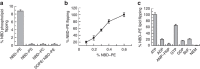
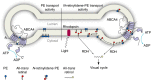
ATP-binding cassette (ABC) transporters comprise a superfamily of proteins, which actively transport a variety of compounds across cell membranes. Mammalian and most eukaryotic ABC transporters function as exporters, flipping or extruding substrates from the cytoplasmic to the extracellular or lumen side of cell membranes. Prokaryotic ABC transporters function either as exporters or importers. Here we show that ABCA4, an ABC transporter found in retinal photoreceptor cells and associated with Stargardt macular degeneration, is a novel importer that actively flips N-retinylidene-phosphatidylethanolamine from the lumen to the cytoplasmic leaflet of disc membranes, thereby facilitating the removal of potentially toxic retinoid compounds from photoreceptors. ABCA4 also actively transports phosphatidylethanolamine in the same direction. Mutations known to cause Stargardt disease decrease N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine transport activity of ABCA4. These studies provide the first direct evidence for a mammalian ABC transporter that functions as an importer and provide insight into mechanisms underlying substrate transport and the molecular basis of Stargardt disease.
Figures




References
-
- Hollenstein K., Dawson R. J. & Locher K. P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 17, 412–418 (2007). - PubMed
-
- Yazaki K., Shitan N., Sugiyama A. & Takanashi K. Cell and molecular biology of ATP-binding cassette proteins in plants. Int. Rev. Cell. Mol. Biol. 276, 263–299 (2009). - PubMed
-
- Borst P. & Elferink R. O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71, 537–592 (2002). - PubMed
-
- Dawson R. J., Hollenstein K. & Locher K. P. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 65, 250–257 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

