Cone outer segments: a biophysical model of membrane dynamics, shape retention, and lamella formation
- PMID: 22735519
- PMCID: PMC3379012
- DOI: 10.1016/j.bpj.2012.04.052
Cone outer segments: a biophysical model of membrane dynamics, shape retention, and lamella formation
Abstract
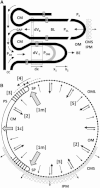
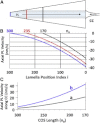
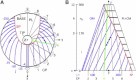
An hypothesis is developed to explain how the unique, right circular conical geometry of cone outer segments (COSs) in Xenopus laevis and other lower vertebrates is maintained during the cycle of axial shortening by apical phagocytosis and axial elongation via the addition of new basal lamellae. Extension of a new basal evagination (BE) applies radial (lateral) traction to membrane and cytoplasmic domains, achieving two coupled effects. 1), The bilayer domain is locally stretched/dilated, creating an entropic driving force that draws membrane components into the BE from the COS's distributed bilayer phase, i.e., plasmalemma and older lamellae (membrane recycling). Membrane proteins, e.g., opsins, are carried passively in this advective, bilayer-driven process. 2), With BE stretching, hydrostatic pressure within the BE cytoplasm is reduced slightly with respect to that of the axonemal cytoplasmic reservoir, allowing cytoplasmic flow into the BE. Attendant lowering of the reservoir's hydrostatic pressure facilitates the subsequent transfer of cytoplasm from lamellar domains to the reservoir (cytoplasmic recycling). The geometry of the BE reflects the membrane/cytoplasm ratio needed for its construction, and essentially specifies the ratio of components recycled from older lamellae. Length and taper angle of the COS reflect the ratio of recycled/new components constructing a new BE. The model also integrates the trajectories and dynamics of lamella open margin lattice components. Although not fully evaluated, the initial model has been assessed against the relevant literature, and three experimental predictions are derived.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Nilsson S.E.G. The ultrastructure of the receptor outer segments in the retina of the leopard frog (Rana pipiens) J. Ultrastruct. Res. 1965;12:207–231. - PubMed
-
- Fetter R.D., Corless J.M. Morphological components associated with frog cone outer segment disc margins. Invest. Ophthalmol. Vis. Sci. 1987;28:646–657. - PubMed
-
- Corless J.M., Worniałło E., Fetter R.D. Modulation of disk margin structure during renewal of cone outer segments in the vertebrate retina. J. Comp. Neurol. 1989;287:531–544. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

