Equilibrium unfolding of the PDZ domain of β2-syntrophin
- PMID: 22735534
- PMCID: PMC3379018
- DOI: 10.1016/j.bpj.2012.05.021
Equilibrium unfolding of the PDZ domain of β2-syntrophin
Abstract
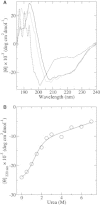
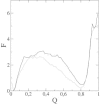
β2-syntrophin, a dystrophin-associated protein, plays a pivotal role in insulin secretion by pancreatic β-cells. It contains a PDZ domain (β2S-PDZ) that, in complex with protein-tyrosine phosphatase ICA512, anchors the dense insulin granules to actin filaments. The phosphorylation state of β2-syntrophin allosterically regulates the affinity of β2S-PDZ for ICA512, and the disruption of the complex triggers the mobilization of the insulin granule stores. Here, we investigate the thermal unfolding of β2S-PDZ at different pH and urea concentrations. Our results indicate that, unlike other PDZ domains, β2S-PDZ is marginally stable. Thermal denaturation experiments show broad transitions and cold denaturation, and a two-state model fit reveals a significant unfolded fraction under physiological conditions. Furthermore, T(m) and T(max) denaturant-dependent shifts and noncoincidence of melting curves monitored at different wavelengths suggest that two-state and three-state models fail to explain the equilibrium data properly and are in better agreement with a downhill scenario. Its higher stability at pH >9 and the results of molecular dynamics simulations indicate that this behavior of β2S-PDZ might be related to its charge distribution. All together, our results suggest a link between the conformational plasticity of the native ensemble of this PDZ domain and the regulation of insulin secretion.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Adams M.E., Dwyer T.M., Froehner S.C. Mouse α1- and β2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J. Biol. Chem. 1995;270:25859–25865. - PubMed
-
- Ort T., Maksimova E., Solimena M. The receptor tyrosine phosphatase-like protein ICA512 binds the PDZ domains of β2-syntrophin and nNOS in pancreatic β-cells. Eur. J. Cell Biol. 2000;79:621–630. - PubMed
-
- Lumeng C., Phelps S., Chamberlain J.S. Interactions between β2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat. Neurosci. 1999;2:611–617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

