Homotrimer formation and dissociation of pharaonis halorhodopsin in detergent system
- PMID: 22735541
- PMCID: PMC3379016
- DOI: 10.1016/j.bpj.2012.05.008
Homotrimer formation and dissociation of pharaonis halorhodopsin in detergent system
Abstract
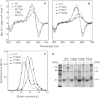
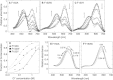
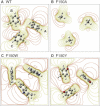
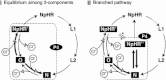
Halorhodopsin from NpHR is a light-driven Cl(-) pump that forms a trimeric NpHR-bacterioruberin complex in the native membrane. In the case of NpHR expressed in Escherichia coli cell, NpHR forms a robust homotrimer in a detergent DDM solution. To identify the important residue for the homotrimer formation, we carried out mutation experiments on the aromatic amino acids expected to be located at the molecular interface. The results revealed that Phe(150) was essential to form and stabilize the NpHR trimer in the DDM solution. Further analyses for examining the structural significance of Phe(150) showed the dissociation of the trimer in F150A (dimer) and F150W (monomer) mutants. Only the F150Y mutant exhibited dissociation into monomers in an ionic strength-dependent manner. These results indicated that spatial positions and interactions between F150-aromatic side chains were crucial to homotrimer stabilization. This finding was supported by QM calculations. In a functional respect, differences in the reaction property in the ground and photoexcited states were revealed. The analysis of photointermediates revealed a decrease in the accumulation of O, which is important for Cl(-) release, and the acceleration of the decay rate in L1 and L2, which are involved in Cl(-) transfer inside the molecule, in the trimer-dissociated mutants. Interestingly, the affinity of them to Cl(-) in the photoexcited state increased rather than the trimer, whereas that in the ground state was almost the same without relation to the oligomeric state. It was also observed that the efficient recovery of the photocycle to the ground state was inhibited in the mutants. In addition, a branched pathway that was not included in Cl(-) transportation was predicted. These results suggest that the trimer assembly may contribute to the regulation of the dynamics in the excited state of NpHR.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Effect of chloride binding on the thermal trimer-monomer conversion of halorhodopsin in the solubilized system.Biochemistry. 2009 Dec 29;48(51):12089-95. doi: 10.1021/bi901380c. Biochemistry. 2009. PMID: 19938870
-
Halorhodopsin from natronomonas pharaonis forms a trimer even in the presence of a detergent, dodecyl-beta-D-maltoside.Photochem Photobiol. 2009 Jan-Feb;85(1):130-6. doi: 10.1111/j.1751-1097.2008.00406.x. Epub 2008 Aug 12. Photochem Photobiol. 2009. PMID: 18700862
-
Probing the Cl--pumping photocycle of pharaonis halorhodopsin: Examinations with bacterioruberin, an intrinsic dye, and membrane potential-induced modulation of the photocycle.Biochim Biophys Acta. 2015 Aug;1847(8):748-58. doi: 10.1016/j.bbabio.2015.05.002. Epub 2015 May 8. Biochim Biophys Acta. 2015. PMID: 25960108
-
Microbial Halorhodopsins: Light-Driven Chloride Pumps.Chem Rev. 2018 Nov 14;118(21):10629-10645. doi: 10.1021/acs.chemrev.7b00715. Epub 2018 Jun 8. Chem Rev. 2018. PMID: 29882660 Review.
-
Halorhodopsin: light-driven ion pumping made simple?Curr Opin Struct Biol. 2002 Aug;12(4):516-22. doi: 10.1016/s0959-440x(02)00356-1. Curr Opin Struct Biol. 2002. PMID: 12163076 Review.
Cited by
-
Functional importance of the oligomer formation of the cyanobacterial H+ pump Gloeobacter rhodopsin.Sci Rep. 2019 Jul 24;9(1):10711. doi: 10.1038/s41598-019-47178-5. Sci Rep. 2019. PMID: 31341208 Free PMC article.
-
Spectroscopic Characterization of Halorhodopsin Reconstituted into Nanodisks Using Native Lipids.Biophys J. 2020 Jun 2;118(11):2853-2865. doi: 10.1016/j.bpj.2020.04.021. Epub 2020 Apr 29. Biophys J. 2020. PMID: 32396848 Free PMC article.
-
Presence of a Haloarchaeal Halorhodopsin-Like Cl- Pump in Marine Bacteria.Microbes Environ. 2018 Mar 29;33(1):89-97. doi: 10.1264/jsme2.ME17197. Epub 2018 Mar 16. Microbes Environ. 2018. PMID: 29553064 Free PMC article.
References
-
- Spudich J.L., Yang C.S., Spudich E.N. Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 2000;16:365–392. - PubMed
-
- Kolbe M., Besir H., Oesterhelt D. Structure of the light-driven chloride pump halorhodopsin at 1.8 A resolution. Science. 2000;288:1390–1396. - PubMed
-
- Kouyama T., Kanada S., Ihara K. Crystal structure of the light-driven chloride pump halorhodopsin from Natronomonas pharaonis. J. Mol. Biol. 2010;396:564–579. - PubMed
-
- Gordeliy V.I., Labahn J., Engelhard M. Molecular basis of transmembrane signalling by sensory rhodopsin II-transducer complex. Nature. 2002;419:484–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

