Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours
- PMID: 22735906
- PMCID: PMC3405214
- DOI: 10.1038/bjc.2012.274
Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours
Abstract
Background: TAS-102 consists of α, α, α-trifluorothymidine (TFT) and an inhibitor of thymidine phosphorylase (TPI). We conducted a dose-escalation phase I study in Japanese patients with advanced solid tumours.
Methods: TAS-102 was administered twice daily on days 1-5 and days 8-12 in a 28-day cycle to patients with solid tumours refractory to standard chemotherapy, to determine its maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and pharmacokinetics (PKs). MTD was evaluated in cycle 1.
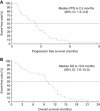
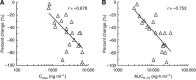
Results: Safety and PKs were evaluated in 21 patients treated with TAS-102 at 30, 40, 50, 60, or 70 mg m(-2) per day. DLTs, such as grade 4 leucopenia, grade 4 neutropenia, and grade 4 thrombocytopenia, were observed in two patients at doses of 30 and 70 mg m(-2). α, α, α-trifluorothymidine and TPI exposures increased dose dependently, and the percentage of decrease in neutrophil count and TFT exposure were significantly correlated. The disease control rate was 50.0% with a median progression-free survival of 2.4 months in 18 colorectal cancer patients. The dose of TAS-102 was not increased above 70 mg m(-2) per day because of the increased tendency for grade 3 and 4 neutropenia, and 70 mg m(-2) per day was the recommended dose for phase II studies.
Conclusions: TAS-102 at 70 mg m(-2) per day was tolerated in Japanese patients with advanced solid tumours. Phase II studies are ongoing in patients with colorectal cancer.
© 2012 Cancer Research UK
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ansfield FJ, Ramirez G (1971) Phase I and II studies of 2'-deoxy-5-(trifluoromethyl)-uridine (NSC-75520). Cancer Chemother Rep 55: 205–208 - PubMed
-
- Emura T, Nakagawa F, Fujioka A, Ohshimo H, Yokogawa T, Okabe H, Kitazato K (2004a) An optimal dosing schedule for a novel combination antimetabolite, TAS-102, based on its intracellular metabolism and its incorporation into DNA. Int J Mol Med 13: 249–255 - PubMed
-
- Emura T, Suzuki N, Yamaguchi M, Ohshimo H, Fukushima M (2004b) A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol 25: 571–578 - PubMed
-
- Fujiwara Y, Heidelberger C (1970a) Fluorinated pyrimidines. XXXVII. The incorporation of 5-trifluoromethyl-2'-deoxyuridine into the deoxyribonucleic acid vaccinia virus. Mol Pharmacol 6: 281–291 - PubMed
-
- Fujiwara Y, Oki T, Heidelberger C (1970b) Fluorinated pyrimidines. XXXVII. Effect of 5-trifluoromethyl-2'-deoxyuridine on the synthesis of deoxyribonucleic acid of mammalian cells in culture. Mol Pharmacol 6: 273–280 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

