The impact of population-based faecal occult blood test screening on colorectal cancer mortality: a matched cohort study
- PMID: 22735907
- PMCID: PMC3394992
- DOI: 10.1038/bjc.2012.277
The impact of population-based faecal occult blood test screening on colorectal cancer mortality: a matched cohort study
Abstract
Background: Randomised trials show reduced colorectal cancer (CRC) mortality with faecal occult blood testing (FOBT). This outcome is now examined in a routine, population-based, screening programme.
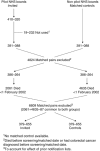
Methods: Three biennial rounds of the UK CRC screening pilot were completed in Scotland (2000-2007) before the roll out of a national programme. All residents (50-69 years) in the three pilot Health Boards were invited for screening. They received a FOBT test by post to complete at home and return for analysis. Positive tests were followed up with colonoscopy. Controls, selected from non-pilot Health Boards, were matched by age, gender, and deprivation and assigned the invitation date of matched invitee. Follow-up was from invitation date to 31 December 2009 or date of death if earlier.
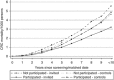
Results: There were 379 655 people in each group (median age 55.6 years, 51.6% male). Participation was 60.6%. There were 961 (0.25%) CRC deaths in invitees, 1056 (0.28%) in controls, rate ratio (RR) 0.90 (95% confidence interval (CI) 0.83-0.99) overall and 0.73 (95% CI 0.65-0.82) for participants. Non-participants had increased CRC mortality compared with controls, RR 1.21 (95% CI 1.06-1.38).
Conclusion: There was a 10% relative reduction in CRC mortality in a routine screening programme, rising to 27% in participants.
Figures


References
-
- Aalen OO (1978) Non-parametric inference for a family of counting processes. Ann Statis 6: 701–726
-
- Cuzick J, Edwards R, Segnan N (1997) Adjusting for non-compliance and contamination in randomised clinical trials. Stat Med 16: 1017–1029 - PubMed
-
- Faivre J, Dancourt V, Lejeune C, Tuzi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C (2004) Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 126: 1674–1680 - PubMed
-
- Hardcastle JD, Chamberlain JO, Robinson MHE, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM (1996) Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 348: 1472–1477 - PubMed
-
- Kewenter J, Brevinge H, Engaras B, Haglind E, Ahran C (1994) Results of screening, rescreening and follow-up in a prospective randomised study for detection of colorectal cancer by fecal occult blood testing. Scand J Gastroenterol 29: 468–473 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

