Paradoxically increased FOXP3+ T cells in IBD do not preferentially express the isoform of FOXP3 lacking exon 2
- PMID: 22736020
- PMCID: PMC3482978
- DOI: 10.1007/s10620-012-2292-3
Paradoxically increased FOXP3+ T cells in IBD do not preferentially express the isoform of FOXP3 lacking exon 2
Abstract
Background: Forkhead box P3 (FOXP3)+ regulatory T cells (Tregs) are critical for controlling inflammation in the gastrointestinal tract. There is a paradoxical increase of mucosal FOXP3+ T cells in patients with inflammatory bowel disease (IBD). These FOXP3+ cells were recently shown to include interleukin (IL)-17A-producing cells in Crohn's disease, resembling Th17 cells implicated in autoimmune diseases. FOXP3 inhibits IL-17A production, but a naturally occurring splice variant of FOXP3 lacking exon 2 (Δexon2) cannot.
Aims: We hypothesized that IBD patients preferentially express the Δexon2 variant of FOXP3 so the paradoxically increased mucosal Tregs in IBD could represent cells expressing only Δexon2.
Methods: We used antibodies and primers that can distinguish between the full-length and Δexon2 splice variant of FOXP3 to evaluate expression of these isoforms in human intestinal tissue by immunohistochemistry and quantitative polymerase chain reaction (PCR), respectively.
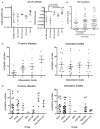
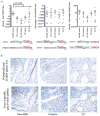
Results: No difference in the expression pattern of Δexon2 relative to full-length FOXP3 was seen in ulcerative colitis or Crohn's disease versus non-IBD controls. By immunofluorescence microscopy and flow cytometry, we also did not find individual cells which expressed FOXP3 protein exclusively in the Δexon2 isoform in either IBD or control tissue. FOXP3+ mucosal CD4+ T cells from both IBD and control specimens were able to make IL-17A in vitro after phorbol myristate acetate (PMA) and ionomycin stimulation, but these cells did not preferentially express Δexon2.
Conclusions: Our data do not support the hypothesis that selective expression of FOXP3 in the Δexon2 isoform accounts for the inability of copious FOXP3+ T cells to inhibit inflammation or IL-17 expression in IBD.
Figures



References
-
- Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. - PubMed
-
- Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. - PubMed
-
- Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

