The PRINTO criteria for clinically inactive disease in juvenile dermatomyositis
- PMID: 22736096
- PMCID: PMC5040631
- DOI: 10.1136/annrheumdis-2012-201483
The PRINTO criteria for clinically inactive disease in juvenile dermatomyositis
Abstract
Objectives: To develop data-driven criteria for clinically inactive disease on and off therapy for juvenile dermatomyositis (JDM).
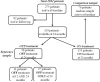
Methods: The Paediatric Rheumatology International Trials Organisation (PRINTO) database contains 275 patients with active JDM evaluated prospectively up to 24 months. Thirty-eight patients off therapy at 24 months were defined as clinically inactive and included in the reference group. These were compared with a random sample of 76 patients who had active disease at study baseline. Individual measures of muscle strength/endurance, muscle enzymes, physician's and parent's global disease activity/damage evaluations, inactive disease criteria derived from the literature and other ad hoc criteria were evaluated for sensitivity, specificity and Cohen's κ agreement.
Results: The individual measures that best characterised inactive disease (sensitivity and specificity >0.8 and Cohen's κ >0.8) were manual muscle testing (MMT) ≥78, physician global assessment of muscle activity=0, physician global assessment of overall disease activity (PhyGloVAS) ≤0.2, Childhood Myositis Assessment Scale (CMAS) ≥48, Disease Activity Score ≤3 and Myositis Disease Activity Assessment Visual Analogue Scale ≤0.2. The best combination of variables to classify a patient as being in a state of inactive disease on or off therapy is at least three of four of the following criteria: creatine kinase ≤150, CMAS ≥48, MMT ≥78 and PhyGloVAS ≤0.2. After 24 months, 30/31 patients (96.8%) were inactive off therapy and 69/145 (47.6%) were inactive on therapy.
Conclusion: PRINTO established data-driven criteria with clearly evidence-based cut-off values to identify JDM patients with clinically inactive disease. These criteria can be used in clinical trials, in research and in clinical practice.
References
-
- Huber A, Feldman BM. Long-term outcomes in juvenile dermatomyositis: how did we get here and where are we going? Curr Rheumatol Rep. 2005;7:441–6. - PubMed
-
- Ravelli A, Trail L, Ferrari C, et al. Long-term outcome and prognostic factors of juvenile dermatomyositis: A multinational, multicenter study of 490 patients. Arthritis Care Res (Hoboken ) 2010;62:63–72. - PubMed
-
- Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical