Mediators of a long-term movement abnormality in a Drosophila melanogaster model of classic galactosemia
- PMID: 22736462
- PMCID: PMC3484862
- DOI: 10.1242/dmm.009050
Mediators of a long-term movement abnormality in a Drosophila melanogaster model of classic galactosemia
Abstract
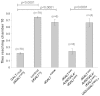
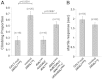
Despite neonatal diagnosis and life-long dietary restriction of galactose, many patients with classic galactosemia grow to experience significant long-term complications. Among the more common are speech, cognitive, behavioral, ovarian and neurological/movement difficulties. Despite decades of research, the pathophysiology of these long-term complications remains obscure, hindering prognosis and attempts at improved intervention. As a first step to overcome this roadblock we have begun to explore long-term outcomes in our previously reported GALT-null Drosophila melanogaster model of classic galactosemia. Here we describe the first of these studies. Using a countercurrent device, a simple climbing assay, and a startle response test to characterize and quantify an apparent movement abnormality, we explored the impact of cryptic GALT expression on phenotype, tested the role of sublethal galactose exposure and galactose-1-phosphate (gal-1P) accumulation, tested the impact of age, and searched for potential anatomical defects in brain and muscle. We found that about 2.5% residual GALT activity was sufficient to reduce outcome severity. Surprisingly, sublethal galactose exposure and gal-1P accumulation during development showed no effect on the adult phenotype. Finally, despite the apparent neurological or neuromuscular nature of the complication we found no clear morphological differences between mutants and controls in brain or muscle, suggesting that the defect is subtle and/or is physiologic rather than structural. Combined, our results confirm that, like human patients, GALT-null Drosophila experience significant long-term complications that occur independently of galactose exposure, and serve as a proof of principle demonstrating utility of the GALT-null Drosophila model as a tool for exploring genetic and environmental modifiers of long-term outcome in GALT deficiency.
Figures




References
-
- Akai S. (1979). Genetic variation in walking ability of Drosophila melanogaster. Jpn. J. Genet. 54, 317–324
-
- Beutler E., Baluda M. L., Sturgeon P., Day R. W. (1965). A new genetic abnormality resulting in galactose-1-phosphate uridyltransferase deficiency. Lancet 1, 353–354 - PubMed
-
- Fridovich-Keil J. L., Walter J. H. (2008). Galactosemia. In The Online Metabolic & Molecular Bases of Inherited Disease (ed. Valle D., Beaudet A., Vogelstein B., Kinzler K., Antonarakis S., Ballabio A.), part 7, ch. 72. New York: McGraw Hill
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

