Treating oral mucositis with a supersaturated calcium phosphate rinse: comparison with control in patients undergoing allogeneic hematopoietic stem cell transplantation
- PMID: 22736463
- PMCID: PMC3411282
- DOI: 10.1007/s00520-012-1489-5
Treating oral mucositis with a supersaturated calcium phosphate rinse: comparison with control in patients undergoing allogeneic hematopoietic stem cell transplantation
Abstract
Purpose: Of patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT), 75% or more experience oral mucositis, a painful acute complication that can delay discharge, interrupt treatment, and threaten life. To evaluate the efficacy of a supersaturated calcium phosphate rinse (SCPR), we compared it with customary care--topical mouth solutions--on measures of severity and consequent interventions and complications.
Methods: In this randomized controlled trial, 40 patients undergoing allogeneic HSCT were randomized: 20 to SCPR four times daily and 20 to solutions made with salvia leaf extract, iodine-povidine, and fluconazole. Treatment extended from initiation of conditioning treatment until the granulocyte count was ≥0.2 g/L. Mucositis severity was measured daily by a hematologist according to a World Health Organization (WHO) scale and self-assessed by patients. Need for interventions [analgesics, total parenteral nutrition (TPN), and granulocyte colony-stimulating factor] and complications (acute graft-versus-host disease and infections) were also assessed.
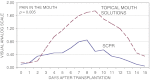
Results: In comparison with the control group, the SCPR group had significantly lower mean measures of WHO oral toxicity (0.9 vs. 1.8; P = 0.02), disease course (3.2 vs. 7.1 days; P = 0.02), and peak mouth pain (0.85 vs. 1.75; P = 0.005). Analgesic need was significantly shorter (1.1 vs. 3.4 days; P = 0.047) and the need for TPN significantly lower (0 vs. 6 patients; P = 0.02; 0 vs. 1.9 mean days; P = 0.009). Measures of complications were lower in the SCPR group, but not significantly so. Trial limitations include the impracticality of achieving double blinding with agents so different in appearance and in preadministration preparation.
Conclusions: Compared with the control group, the SCPR group had significantly lower mean measures of oral toxicity, peak mouth pain, and disease course duration. These results warrant confirmation in controlled, multicenter, randomized trials.
Figures
References
-
- Wardley AM, Jayson GC, Swindell R, Morgenstern GR, Chang J, Bloor R, Fraser CJ, Scarffe JH. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. Br J Haematol. 2000;110:292–299. doi: 10.1046/j.1365-2141.2000.02202.x. - DOI - PubMed
-
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. - DOI - PubMed
-
- Epstein JB, Schubert MM. Oropharyngeal mucositis in cancer therapy. Review of pathogenesis, diagnosis, and management. Oncology (Williston Park) 2003;17:1767–1779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials