Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis
- PMID: 22736750
- PMCID: PMC4029334
- DOI: 10.1177/1352458512451510
Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis
Abstract
Background: It is unclear if all patients with relapsing-remitting multiple sclerosis (RRMS) ultimately develop progressive MS. Onset of progressive disease course seems to be age- rather than disease duration-dependent. Some forms of progressive MS (e.g. primary progressive MS (PPMS)) are uncommon in population-based studies. Ascertainment of patients with PPMS from clinic-based populations can facilitate a powerful comparison of age at progression onset between secondary progressive MS (SPMS) and PPMS but may introduce unclear biases.
Objective: Our aim is to confirm that onset of progressive disease course is more relevant to the patient's age than the presence or duration of a pre-progression relapsing disease course in MS.
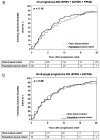
Methods: We studied a population-based MS cohort (n=210, RRMS n=109, progressive MS n=101) and a clinic-based progressive MS cohort (n=754). Progressive course was classified as primary (PPMS; n=322), single attack (SAPMS; n=112) and secondary progressive (SPMS; n=421). We studied demographics (chi(2) or t-test), age-of-progression-onset (t-test) and time to Expanded Disability Status Scale of 6 (EDSS6) (Kaplan-Meier analyses).
Results: Sex ratio (p=0.58), age at progression onset (p=0.37) and time to EDSS6 (p=0.16) did not differ between the cohorts. Progression had developed before age 75 in 99% of patients with known progressive disease course; 38% with RRMS did not develop progression by age 75. Age at progression onset did not differ between SPMS (44.9±9.6), SAPMS (45.5±9.6) and PPMS (45.7±10.8). In either cohort, only 2% of patients had reached EDSS6 before onset of progression.
Conclusions: Patients with RRMS do not inevitably develop a progressive disease course. Onset of progression is more dependent on age than the presence or duration of a pre-progression symptomatic disease course. Moderate disability is sustained predominantly after the onset of a progressive disease course in MS.
Conflict of interest statement
Figures



References
-
- Kantarci OH, Weinshenker BG. Natural history of multiple sclerosis. Neurologic Clinics. 2005;23(1):17–38. - PubMed
-
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129(Pt 3):606–16. - PubMed
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907–11. - PubMed
-
- Wolinsky JS. The diagnosis of primary progressive multiple sclerosis. Journal of the neurological sciences. 2003;206(2):145–52. - PubMed
-
- Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584–94. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS032129/NS/NINDS NIH HHS/United States
- U01NS 45719/NS/NINDS NIH HHS/United States
- R01 NS058698/NS/NINDS NIH HHS/United States
- P50 NS038667/NS/NINDS NIH HHS/United States
- R01 NS048357/NS/NINDS NIH HHS/United States
- UL1 RR024150/RR/NCRR NIH HHS/United States
- 2P50NS038667-11/NS/NINDS NIH HHS/United States
- U01 NS045719/NS/NINDS NIH HHS/United States
- R01 NS065829/NS/NINDS NIH HHS/United States
- R01 NS060881/NS/NINDS NIH HHS/United States
- R01 NS 49577-6/NS/NINDS NIH HHS/United States
- R01 NS 58698/NS/NINDS NIH HHS/United States
- R01 NS058698-01A2/NS/NINDS NIH HHS/United States
- UL1 RR 24150-2/RR/NCRR NIH HHS/United States
- R01 NS65829/NS/NINDS NIH HHS/United States
- R01 NS049577/NS/NINDS NIH HHS/United States
- R21 NS073684/NS/NINDS NIH HHS/United States
- R01 GM092993/GM/NIGMS NIH HHS/United States
- R01 NS 60881/NS/NINDS NIH HHS/United States
- R01 NS060881-01A209/NS/NINDS NIH HHS/United States
- R01 NS024180/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

