Modular dispensability of dysferlin C2 domains reveals rational design for mini-dysferlin molecules
- PMID: 22736764
- PMCID: PMC3431656
- DOI: 10.1074/jbc.M112.391722
Modular dispensability of dysferlin C2 domains reveals rational design for mini-dysferlin molecules
Retraction in
-
Modular dispensability of dysferlin C2 domains reveals rational design for mini-dysferlin molecules.J Biol Chem. 2017 Jul 28;292(30):12543. doi: 10.1074/jbc.A112.391722. J Biol Chem. 2017. PMID: 28754768 Free PMC article. No abstract available.
Abstract
Dysferlin is a large transmembrane protein composed of a C-terminal transmembrane domain, two DysF domains, and seven C2 domains that mediate lipid- and protein-binding interactions. Recessive loss-of-function mutations in dysferlin lead to muscular dystrophies, for which no treatment is currently available. The large size of dysferlin precludes its encapsulation into an adeno-associated virus (AAV), the vector of choice for gene delivery to muscle. To design mini-dysferlin molecules suitable for AAV-mediated gene transfer, we tested internally truncated dysferlin constructs, each lacking one of the seven C2 domains, for their ability to localize to the plasma membrane and to repair laser-induced plasmalemmal wounds in dysferlin-deficient human myoblasts. We demonstrate that the dysferlin C2B, C2C, C2D, and C2E domains are dispensable for correct plasmalemmal localization. Furthermore, we show that the C2B, C2C, and C2E domains and, to a lesser extent, the C2D domain are dispensable for dysferlin membrane repair function. On the basis of these results, we designed small dysferlin molecules that can localize to the plasma membrane and reseal laser-induced plasmalemmal injuries and that are small enough to be incorporated into AAV. These results lay the groundwork for AAV-mediated gene therapy experiments in dysferlin-deficient mouse models.
Figures




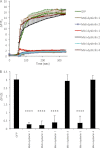
References
-
- Therrien C., Di Fulvio S., Pickles S., Sinnreich M. (2009) Characterization of lipid binding specificities of dysferlin C2 domains reveals novel interactions with phosphoinositides. Biochemistry 48, 2377–2384 - PubMed
-
- Davis D. B., Doherty K. R., Delmonte A. J., McNally E. M. (2002) Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J. Biol. Chem. 277, 22883–22888 - PubMed
-
- Lennon N. J., Kho A., Bacskai B. J., Perlmutter S. L., Hyman B. T., Brown R. H., Jr. (2003) Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J. Biol. Chem. 278, 50466–50473 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

