SplicerEX: a tool for the automated detection and classification of mRNA changes from conventional and splice-sensitive microarray expression data
- PMID: 22736799
- PMCID: PMC3404365
- DOI: 10.1261/rna.033621.112
SplicerEX: a tool for the automated detection and classification of mRNA changes from conventional and splice-sensitive microarray expression data
Abstract
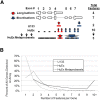
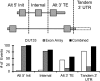
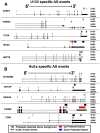
The key postulate that one gene encodes one protein has been overhauled with the discovery that one gene can generate multiple RNA transcripts through alternative mRNA processing. In this study, we describe SplicerEX, a novel and uniquely motivated algorithm designed for experimental biologists that (1) detects widespread changes in mRNA isoforms from both conventional and splice sensitive microarray data, (2) automatically categorizes mechanistic changes in mRNA processing, and (3) mitigates known technological artifacts of exon array-based detection of alternative splicing resulting from 5' and 3' signal attenuation, background detection limits, and saturation of probe set signal intensity. In this study, we used SplicerEX to compare conventional and exon-based Affymetrix microarray data in a model of EBV transformation of primary human B cells. We demonstrated superior detection of 3'-located changes in mRNA processing by the Affymetrix U133 GeneChip relative to the Human Exon Array. SplicerEX-identified exon-level changes in the EBV infection model were confirmed by RT-PCR and revealed a novel set of EBV-regulated mRNA isoform changes in caspases 6, 7, and 8. Finally, SplicerEX as compared with MiDAS analysis of publicly available microarray data provided more efficiently categorized mRNA isoform changes with a significantly higher proportion of hits supported by previously annotated alternative processing events. Therefore, SplicerEX provides an important tool for the biologist interested in studying changes in mRNA isoform usage from conventional or splice-sensitive microarray platforms, especially considering the expansive amount of archival microarray data generated over the past decade. SplicerEX is freely available upon request.
Figures



References
-
- Affymetrix. 2005. Alternative transcript analysis methods for exon arrays. Affymetrix Whitepaper 2005. http://www.affymetrix.com/support/technical/whitepapers.affx.
-
- Blencowe BJ 2006. Alternative splicing: New insights from global analyses. Cell 126: 37–47 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
