Identification of a secreted fatty acid and retinol-binding protein (Hp-FAR-1) from Heligmosomoides polygyrus
- PMID: 22736819
- PMCID: PMC3380493
Identification of a secreted fatty acid and retinol-binding protein (Hp-FAR-1) from Heligmosomoides polygyrus
Abstract
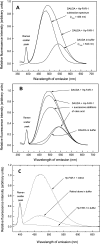
Hp-FAR-1 is a major, secreted antigen of the parasitic nematode Heligmosomoides polygyrus, a laboratory mouse model frequently used to study the cellular mechanisms of chronic helminth infections. The DNA encoding Hp-FAR-1 was recovered by screening a fourth larval (L₄) H. polygyrus cDNA expression library using antibodies raised against L₄ stage excretory/secretory (E/S) proteins. Predictions of secondary structure based on the Hp-FAR-1 amino acid sequence indicated that an alpha-helix predominates in Hp-FAR-1, possibly with some coiled-coil conformation, with no beta-structure. Fluorescence-based ligand binding analysis confirmed that the recombinant Hp-FAR-1 (rHp-FAR-1) binds the fluorescent fatty acid analog 11-((5-[dimethylaminoaphthalene-1-sulfonyl)amino)undecanoic acid (DAUDA), and by competition oleic acid. RT-PCR amplification of the hp-far-1 gene indicated that the gene is transcribed in all parasitic stages of the organism's life cycle. The presence of a secreted FAR protein in the well-defined laboratory model of H. polygyrus provides an excellent model for the further study and analysis of the in vivo role of secreted FAR proteins in parasitism, and supports the mounting evidence that secreted FAR proteins play a major role in nematode parasitism.
Keywords: Heligmosomoides polygyrus; Hp-FAR-1; host-parasitic relationship; hp-far-1; lifecycle; molecular biology; nematode; retinol binding.
Figures


References
-
- Basavaraju S, Zhan B, Kennedy MW, Liu YY, Hawdon J, Hotez PJ. Ac-FAR-1, a 20 kDa fatty acid- and retinol-binding protein secreted by adult Ancylostoma caninum hookworms: gene transcription pattern, ligand binding properties and structural characterisation. Molecular and Biochemical Parasitology. 2003;126:63–71. - PubMed
-
- Behnke JM, Barnard CJ, Wakelin D. Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward. International Journal for Parasitology. 1992;22:861–907. - PubMed
-
- Bjellqvist B, Basse B, Olsen E, Celis JE. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined a pH scale share isoelectric points correlate with polypeptide compositions. Electrophoresis. 1994;15:529–539. - PubMed
-
- Cuff JA, Clamp M, Siddiqui A, Finlay M, Barton GJ. JPred: a consensus secondary structure prediction server. Bioinformatics. 1998;14:892–893. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
