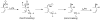Pd and Mo Catalyzed Asymmetric Allylic Alkylation
- PMID: 22736934
- PMCID: PMC3377172
- DOI: 10.1021/op200294r
Pd and Mo Catalyzed Asymmetric Allylic Alkylation
Abstract
The ability to control the alkylation of organic substrates becomes ever more powerful by using metal catalysts. Among the major benefits of metal catalysis is the possibility to perform such processes asymmetrically using only catalytic amounts of the chiral inducing agent which is a ligand to the metal of the catalyst. A unique aspect of asymmetric metal catalyzed processes is the fact that many mechanisms exist for stereoinduction. Furthermore, using the same catalyst system, many types of bonds including but not limited to C-C, C-N, C-O, C-S, C-P, and C-H can be formed asymmetrically. An overview of this process using palladium and molybdenum based metals being developed in my laboratories and how they influence strategy in synthesizing bioactive molecular targets is presented.
Figures
References
-
- Jacobsen EN, Pfaltz A, Yamamoto H, editors. Comprehensive Asymmetric Synthesis. Springer-Berlin; 1999.
- Ojimi I, editor. Catalytic Asymmetric Synthesis. 3. Wiley; New York: 2010.
-
- Knowles WS. Angew Chem Int Ed. 2002;41:1998–2007.
- Noyori R. Angew Chem Int Ed. 2002;41:2008–2022. - PubMed
-
- Sharpless KB. Angew Chem Int Ed. 2002;41:2024–2032. - PubMed
-
- Ooi T, Maruoka K. Angew Chem Int Ed. 2007;46:4222–4266. - PubMed
-
- Dondoni A, Massi A. Angew Chem Int Ed. 2008;47:4638–4660. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous