The link between morphotype transition and virulence in Cryptococcus neoformans
- PMID: 22737071
- PMCID: PMC3380952
- DOI: 10.1371/journal.ppat.1002765
The link between morphotype transition and virulence in Cryptococcus neoformans
Abstract
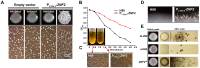
Cryptococcus neoformans is a ubiquitous human fungal pathogen. This pathogen can undergo morphotype transition between the yeast and the filamentous form and such morphological transition has been implicated in virulence for decades. Morphotype transition is typically observed during mating, which is governed by pheromone signaling. Paradoxically, components specific to the pheromone signaling pathways play no or minimal direct roles in virulence. Thus, the link between morphotype transition and virulence and the underlying molecular mechanism remain elusive. Here, we demonstrate that filamentation can occur independent of pheromone signaling and mating, and both mating-dependent and mating-independent morphotype transition require the transcription factor Znf2. High expression of Znf2 is necessary and sufficient to initiate and maintain sex-independent filamentous growth under host-relevant conditions in vitro and during infection. Importantly, ZNF2 overexpression abolishes fungal virulence in murine models of cryptococcosis. Thus, Znf2 bridges the sex-independent morphotype transition and fungal pathogenicity. The impacts of Znf2 on morphological switch and pathogenicity are at least partly mediated through its effects on cell adhesion property. Cfl1, a Znf2 downstream factor, regulates morphogenesis, cell adhesion, biofilm formation, and virulence. Cfl1 is the first adhesin discovered in the phylum Basidiomycota of the Kingdom Fungi. Together with previous findings in other eukaryotic pathogens, our findings support a convergent evolution of plasticity in morphology and its impact on cell adhesion as a critical adaptive trait for pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

