Stretching the rules: monocentric chromosomes with multiple centromere domains
- PMID: 22737088
- PMCID: PMC3380829
- DOI: 10.1371/journal.pgen.1002777
Stretching the rules: monocentric chromosomes with multiple centromere domains
Abstract
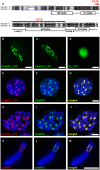
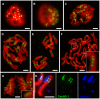
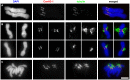
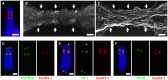
The centromere is a functional chromosome domain that is essential for faithful chromosome segregation during cell division and that can be reliably identified by the presence of the centromere-specific histone H3 variant CenH3. In monocentric chromosomes, the centromere is characterized by a single CenH3-containing region within a morphologically distinct primary constriction. This region usually spans up to a few Mbp composed mainly of centromere-specific satellite DNA common to all chromosomes of a given species. In holocentric chromosomes, there is no primary constriction; the centromere is composed of many CenH3 loci distributed along the entire length of a chromosome. Using correlative fluorescence light microscopy and high-resolution electron microscopy, we show that pea (Pisum sativum) chromosomes exhibit remarkably long primary constrictions that contain 3-5 explicit CenH3-containing regions, a novelty in centromere organization. In addition, we estimate that the size of the chromosome segment delimited by two outermost domains varies between 69 Mbp and 107 Mbp, several factors larger than any known centromere length. These domains are almost entirely composed of repetitive DNA sequences belonging to 13 distinct families of satellite DNA and one family of centromeric retrotransposons, all of which are unevenly distributed among pea chromosomes. We present the centromeres of Pisum as novel "meta-polycentric" functional domains. Our results demonstrate that the organization and DNA composition of functional centromere domains can be far more complex than previously thought, do not require single repetitive elements, and do not require single centromere domains in order to segregate properly. Based on these findings, we propose Pisum as a useful model for investigation of centromere architecture and the still poorly understood role of repetitive DNA in centromere evolution, determination, and function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Jiang JM, Birchler JA, Parrott WA, Dawe RK. A molecular view of plant centromeres. Trends Plant Sci. 2003;8:570–575. - PubMed
-
- Malik HS, Henikoff S. Major evolutionary transitions in centromere complexity. Cell. 2009;138:1067–1082. - PubMed
-
- Black BE, Bassett EA. The histone variant CENP-A and centromere specification. Curr Opin Cell Biol. 2008;20:91–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

