Capnodiaceae
- PMID: 22737101
- PMCID: PMC3377173
- DOI: 10.1007/s13225-011-0145-6
Capnodiaceae
Abstract
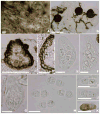
In this paper we revisit the Capnodiaceae with notes on selected genera. Type specimens of the ascomycetous genera Aithaloderma, Anopeltis, Callebaea, Capnodaria, Echinothecium, Phragmocapnias and Scorias were re-examined, described and illustrated. Leptoxyphium is anamorphic Capnodiaceae and Polychaeton is a legitimate and earlier name for Capnodium, but in order to maintain nomenclatural stability we propose that the teleomorphic name should be conisdered for the approved lists of names currently in preparation for fungi. Notes are provided on the ascomycetous genus Scoriadopsis. However, we were unable to locate the type of this genus during the time frame of this study. The ascomycetous genera Aithaloderma, Ceramoclasteropsis, Hyaloscolecostroma and Trichomerium are excluded from Capnodiaceae on the basis of having ascostromata and trans-septate hyaline ascospores and should be accommodated in Chaetothyriaceae. Callebaea is excluded as the ascomata are thyriothecia and the genus is placed in Micropeltidaceae. Echinothecium is excluded as synonym of Sphaerellothecium and is transferred to Mycosphaerellaceae. The type specimen of Capnophaeum is lost and this should be considered as a doubtful genus. The coelomycetous Microxiphium is polyphyletic, while the status of Fumiglobus, Polychaetella and Tripospermum is unclear. Fourteen new collections of sooty moulds made in Thailand were isolated and sequenced. The nuclear large and small rDNA was partially sequenced and compared in a phylogeny used to build a more complete understanding of the relationships of genera in Capnodiaceae. Four new species are described and illustrated, while Phragmocapnias and Scorias are epitypified with fresh collections.
Figures


















References
-
- Andrew JH. Biological control in the phyllosphere. Ann Rev Phytopathol. 1982;30:603–635. - PubMed
-
- Barr ME. Amherst. University of Massachusetts; Massachusetts: 1987. Prodomus to class Loculoascomycetes.
-
- Batista AC, Ciferri R. Capnodiales. Capnodiales. Saccardoa. 1963;2:1–296.
-
- Batista AC, Ciferri R. The Chaetothyriales. Sydowia. 1962;3:1–129.
-
- Batista AC, Ciferri R. The sooty–molds of the family Asbolisiaceae. Quad Ist Bot Univ Lab Crittogam Pavia. 1963;31:1–229.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
