Differential modulation of nitric oxide synthases in aging: therapeutic opportunities
- PMID: 22737132
- PMCID: PMC3382417
- DOI: 10.3389/fphys.2012.00218
Differential modulation of nitric oxide synthases in aging: therapeutic opportunities
Abstract
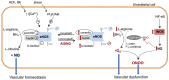
Vascular aging is the term that describes the structural and functional disturbances of the vasculature with advancing aging. The molecular mechanisms of aging-associated endothelial dysfunction are complex, but reduced nitric oxide (NO) bioavailability and altered vascular expression and activity of NO synthase (NOS) enzymes have been implicated as major players. Impaired vascular relaxation in aging has been attributed to reduced endothelial NOS (eNOS)-derived NO, while increased inducible NOS (iNOS) expression seems to account for nitrosative stress and disrupted vascular homeostasis. Although eNOS is considered the main source of NO in the vascular endothelium, neuronal NOS (nNOS) also contributes to endothelial cells-derived NO, a mechanism that is reduced in aging. Pharmacological modulation of NO generation and expression/activity of NOS isoforms may represent a therapeutic alternative to prevent the progression of cardiovascular diseases. Accordingly, this review will focus on drugs that modulate NO bioavailability, such as nitrite anions and NO-releasing non-steroidal anti-inflammatory drugs, hormones (dehydroepiandrosterone and estrogen), statins, resveratrol, and folic acid, since they may be useful to treat/to prevent aging-associated vascular dysfunction. The impact of these therapies on life quality in elderly and longevity will be discussed.
Keywords: aging; endothelial dysfunction; folic acid; nitric oxide; nitric oxide synthases; resveratrol; statin; uncoupled eNOS.
Figures

References
-
- Alp N. J., Mussa S., Khoo J., Cai S., Guzik T., Jefferson A., Goh N., Rockett K. A., Channon K. M. (2003). Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J. Clin. Invest. 112, 725–73510.1172/JCI17786 - DOI - PMC - PubMed
-
- Antoniades C., Bakogiannis C., Leeson P., Guzik T. J., Zhang M. H., Tousoulis D., Antonopoulos A. S., Demosthenous M., Marinou K., Hale A., Paschalis A., Psarros C., Triantafyllou C., Bendall J., Casadei B., Stefanadis C., Channon K. M. (2011). Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation 124, 335–34510.1161/CIRCULATIONAHA.111.029272 - DOI - PMC - PubMed
-
- Antoniades C., Shirodaria C., Warrick N., Cai S., de Bono J., Lee J., Leeson P., Neubauer S., Ratnatunga C., Pillai R., Refsum H., Channon K. M. (2006). 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 114, 1193–120110.1161/CIRCULATIONAHA.106.612325 - DOI - PubMed
-
- Aoki C., Nakano A., Tanaka S., Yanagi K., Ohta S., Jojima T., Kasai K., Takekawa H., Hirata K., Hattori Y. (2012). Fluvastatin upregulates endothelial nitric oxide synthase activity via enhancement of its phosphorylation and expression and via an increase in tetrahydrobiopterin in vascular endothelial cells. Int. J. Cardiol. 156, 55–6110.1016/j.ijcard.2010.10.029 - DOI - PubMed
-
- Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., Simes R. (2005). Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–127810.1016/S0140-6736(05)67394-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources

