Ingestion of Carbohydrate-Rich Supplements during Gestation Programs Insulin and Leptin Resistance but not Body Weight Gain in Adult Rat Offspring
- PMID: 22737135
- PMCID: PMC3382418
- DOI: 10.3389/fphys.2012.00224
Ingestion of Carbohydrate-Rich Supplements during Gestation Programs Insulin and Leptin Resistance but not Body Weight Gain in Adult Rat Offspring
Abstract
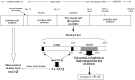
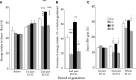
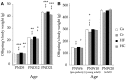
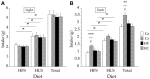
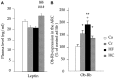
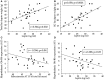
Prenatal nutritional conditions can predispose to development of obesity and metabolic syndrome in adulthood. Gestation with its important modifications in hormonal status is a period of changes in normal feeding habits with pulses of consumption or avoidance of certain categories of food. We tried to mimic in an animal model some changes in food consumption patterns observed in pregnant women. For this purpose, Long-Evans female rats were fed during the dark period, their usual pre-gestational food quantity, and were allowed to complete their daily intake with either a restricted control (Cr), high-fat (HF), or high-carbohydrate (HC) diet available ad libitum during the light period. Dams fed a control diet ad libitum (Ca) served as controls. Body weight and composition, food intake, and metabolic hormones (insulin, leptin) were recorded in male offspring until 20 weeks after birth. Cr and HC females ate less than Ca females (-16%; p < 0.001) and their offspring presented a weight deficit from birth until 6 (HC group) and 10 (Cr group) weeks of age (p < 0.05 or less). Plasma leptin corresponded to low body weight in Cr offspring, but was increased in HC offspring that in addition, had increased plasma insulin, blood glucose, and subcutaneous adipose tissue mass. HF dams ate more than Ca dams (+13%; p < 0.001), but plasma leptin and insulin were similar in their offspring. Hypothalamic Ob-Rb expression was increased in Cr, HC, and HF offspring (+33-100% vs Ca; p < 0.05 or less). HC supplement ingestion during gestation therefore leads to insulin and leptin resistance in adult offspring independently of lower birth weight. These hormonal changes characterize obesity-prone animals. We therefore suggest that attention should be paid to the carbohydrate snacking and overall carbohydrate content in the diet during the last weeks (or months) preceding delivery to limit development of later metabolic disorders in offspring.
Keywords: adipose tissue distribution; dietary preference; fetal programming; ghrelin; high-fat; hypothalamic Ob-Rb expression.
Figures
References
-
- Ahren B., Mansson S., Gingerich R. L., Havel P. J. (1997). Regulation of plasma leptin in mice: influence of age, high-fat diet, and fasting. Am. J. Physiol. 42, R113–R120 - PubMed
-
- Beck B. (2000). “Quantitative and macronutrient-related regulation of hypothalamic neuropeptide Y, galanin and neurotensin,” in Neural and Metabolic Control of Macronutrient Intake, eds Berthoud H. R., Seeley R. J. (Boca Raton, FL: CRC Press; ), 455–464
-
- Beck B. (2005). “The arcuate nucleus: its special place in the central networks that regulate feeding behavior,” in Nutrient and Cell Signalling, ed. Zempleni J. D. K. (New-York: Dekker; ), 665–699
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

