The conductor of the autonomic orchestra
- PMID: 22737143
- PMCID: PMC3380376
- DOI: 10.3389/fendo.2012.00071
The conductor of the autonomic orchestra
Abstract
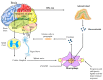
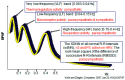
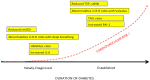
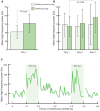
Bad bedfellows - autonomic dysfunction, inflammation, and diabetes! Are they related? How? Evidence suggests the activation of inflammatory cytokines like IL-6 and TNFα in newly diagnosed type 2 diabetes and that the inflammatory change correlates with abnormalities in sympathovagal balance. Dysfunction of the autonomic system predicts cardiovascular risk and sudden death in patients with type 2 diabetes. It occurs in prediabetes, providing opportunities for early intervention. The importance of recognizing autonomic dysfunction as a predictor of morbidity and mortality with intensification of treatment suggests that all patients with type 2 diabetes at onset, and those with type 1 diabetes after 5 years should be screened for autonomic imbalance. These tests can be performed at the bedside with real time output of information - within the scope of the practicing physician - facilitates diagnosis and allows the application of sound strategies for management. The window of opportunity for aggressive control of all the traditional risk factors for cardiovascular events or sudden death with intensification of therapy is with short duration diabetes, the absence of cardiovascular disease, and a history of severe hypoglycemic events. To this list we can now add autonomic dysfunction and neuropathy, which have become the most powerful predictors of risk for mortality. It seems prudent that practitioners should be encouraged to become familiar with this information and apply risk stratification in clinical practice. After all, how difficult is it to ask patients "do you have numb feet?" and to determine their heart rate variability - it could be lifesaving. Ultimately methods to reset the hypothalamus and the inflammatory cascade are needed if we are to impact the care of patients with this compendium of conditions.
Keywords: autonomic; cardiovascular risk; hypothalamic; inflammation.
Figures


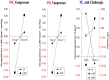
References
-
- Beijers H. J., Ferreira I., Bravenboer B., Dekker J. M., Nijpels G., Heine R. J., Stehouwer C. D. (2009). Microalbuminuria and cardiovascular autonomic dysfunction are independently associated with cardiovascular mortality: evidence for distinct pathways: the Hoorn Study. Diabetes Care 32, 1698–170310.2337/dc08-1544 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

