Pretargeted molecular imaging and radioimmunotherapy
- PMID: 22737190
- PMCID: PMC3364558
- DOI: 10.7150/thno.3582
Pretargeted molecular imaging and radioimmunotherapy
Abstract
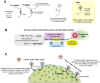
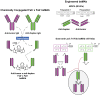
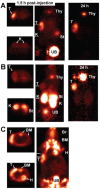
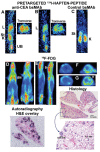
Pretargeting is a multi-step process that first has an unlabeled bispecific antibody (bsMAb) localize within a tumor by virtue of its anti-tumor binding site(s) before administering a small, fast-clearing radiolabeled compound that then attaches to the other portion of the bsMAb. The compound's rapid clearance significantly reduces radiation exposure outside of the tumor and its small size permits speedy delivery to the tumor, creating excellent tumor/nontumor ratios in less than 1 hour. Haptens that bind to an anti-hapten antibody, biotin that binds to streptavidin, or an oligonucleotide binding to a complementary oligonucleotide sequence have all been radiolabeled for use by pretargeting. This review will focus on a highly flexible anti-hapten bsMAb platform that has been used to target a variety of radionuclides to image (SPECT and PET) as well as treat tumors.
Keywords: bispecific antibody; cancer detection; pretargeting; radioimmunodetection; radioimmunotherapy..
Conflict of interest statement
Conflict of Interest: DM Goldenberg, C-H Chang, EA Rossi, and WJ McBride have financial interests in Immunomedics, Inc. or IBC Pharmaceutical, Inc. RM Sharkey declares no conflicts.
Figures

References
-
- Pressman D, Keighley G. The zone of activity of antibodies as determined by the use of radioactive tracers. Fed Proc. 1948;7:308. - PubMed
-
- Pressman D, Korngold L. The in vivo localization of anti-Wagner-osteogenic-sarcoma antibodies. Cancer. 1953;6:619–23. - PubMed
-
- Goldenberg DM, DeLand F, Kim E, Bennett S, Primus FJ, van Nagell JR Jr. et al. Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N Engl J Med. 1978;298:1384–6. - PubMed
-
- Goldenberg DM, Preston DF, Primus FJ, Hansen HJ. Photoscan localization of GW-39 tumors in hamsters using radiolabeled anticarcinoembryonic antigen immunoglobulin G. Cancer Res. 1974;34:1–9. - PubMed
-
- Goldenberg DM, Gaffar SA, Bennett SJ, Beach JL. Experimental radioimmunotherapy of a xenografted human colonic tumor (GW-39) producing carcinoembryonic antigen. Cancer Res. 1981;41:4354–60. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

