Fragile X mental retardation protein interacts with the RNA-binding protein Caprin1 in neuronal RiboNucleoProtein complexes [corrected]
- PMID: 22737234
- PMCID: PMC3380850
- DOI: 10.1371/journal.pone.0039338
Fragile X mental retardation protein interacts with the RNA-binding protein Caprin1 in neuronal RiboNucleoProtein complexes [corrected]
Erratum in
- PLoS One. 2012;7(9). doi:10.1371/annotation/05374d07-34cf-483f-80f4-ec87374cbeb6
Abstract
Fragile X syndrome is caused by the absence of the Fragile X Mental Retardation Protein (FMRP), an RNA-binding protein. FMRP is associated with messenger RiboNucleoParticles (mRNPs) present in polyribosomes and its absence in neurons leads to alteration in synaptic plasticity as a result of translation regulation defects. The molecular mechanisms by which FMRP plays a role in translation regulation remain elusive. Using immunoprecipitation approaches with monoclonal Ab7G1-1 and a new generation of chicken antibodies, we identified Caprin1 as a novel FMRP-cellular partner. In vivo and in vitro evidence show that Caprin1 interacts with FMRP at the level of the translation machinery as well as in trafficking neuronal granules. As an RNA-binding protein, Caprin1 has in common with FMRP at least two RNA targets that have been identified as CaMKIIα and Map1b mRNAs. In view of the new concept that FMRP species bind to RNA regardless of known structural motifs, we propose that protein interactors might modulate FMRP functions.
Conflict of interest statement
Figures




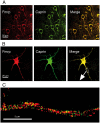
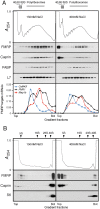
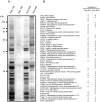

References
-
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–338. - PubMed
-
- Bardoni B, Davidovic L, Bensaid M, Khandjian EW. The fragile X syndrome: exploring its molecular basis and seeking a treatment. Expert Rev Mol Med. 2006;8:1–16. - PubMed
-
- Khandjian EW, Fortin A, Thibodeau A, Tremblay S, Cote F, et al. A heterogeneous set of FMR1 proteins is widely distributed in mouse tissues and is modulated in cell culture. Hum Mol Genet. 1995;4:783–789. - PubMed
-
- Devys D, Lutz Y, Rouyer N, Bellocq J-P, Mandel J-L. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nature Genet. 1993;4:335–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

