Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo
- PMID: 22737239
- PMCID: PMC3380846
- DOI: 10.1371/journal.pone.0039465
Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo
Abstract
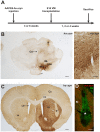
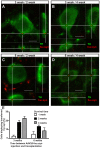
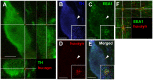
Several people with Parkinson's disease have been treated with intrastriatal grafts of fetal dopaminergic neurons. Following autopsy, 10-22 years after surgery, some of the grafted neurons contained Lewy bodies similar to those observed in the host brain. Numerous studies have attempted to explain these findings in cell and animal models. In cell culture, α-synuclein has been found to transfer from one cell to another, via mechanisms that include exosomal transport and endocytosis, and in certain cases seed aggregation in the recipient cell. In animal models, transfer of α-synuclein from host brain cells to grafted neurons has been shown, but the reported frequency of the event has been relatively low and little is known about the underlying mechanisms as well as the fate of the transferred α-synuclein. We now demonstrate frequent transfer of α-synuclein from a rat brain engineered to overexpress human α-synuclein to grafted dopaminergic neurons. Further, we show that this model can be used to explore mechanisms underlying cell-to-cell transfer of α-synuclein. Thus, we present evidence both for the involvement of endocytosis in α-synuclein uptake in vivo, and for seeding of aggregation of endogenous α-synuclein in the recipient neuron by the transferred α-synuclein. Finally, we show that, at least in a subset of the studied cells, the transmitted α-synuclein is sensitive to proteinase K. Our new model system could be used to test compounds that inhibit cell-to-cell transfer of α-synuclein and therefore might retard progression of Parkinson neuropathology.
Conflict of interest statement
Figures


References
-
- Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci. 2009;1170:615–622. - PubMed
-
- Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann N Y Acad Sci. 2010;1184:55–67. - PubMed
-
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. - PubMed
-
- Kurowska Z, Englund E, Widner H, Lindvall O, Li JY, et al. Signs of Degeneration in 12–22-Year Old Grafts of Mesencephalic Dopamine Neurons in Patients with Parkinson’s Disease. Journal of Parkinson’s Disease. 2011;1:83–92. - PubMed
-
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

