The effects of fumaria parviflora L extract on chronic hand eczema: a randomized double-blind placebo controlled clinical trial
- PMID: 22737422
- PMCID: PMC3371888
The effects of fumaria parviflora L extract on chronic hand eczema: a randomized double-blind placebo controlled clinical trial
Abstract
Background: Hand eczema is a common and distressing condition with multiple causes such as atopy, irritant and allergic contact dermatitis. Fumaria parviflora, is known as Shahtareh in Persian, is a folk medicine for eczema. This study aimed to evaluate the effect of alcoholic extract of Fumaria parviflora on hand eczema.
Methods: In a randomized double-blind, placebo-controlled study, 44 patients with hand eczema were randomly assigned to apply 4% cream of Fumaria parviflora or vehicle cream to hand twice daily for 4 weeks.
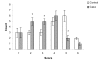
Results: The reduction of eczema area and severity index score before and two weeks after therapy was statistically significant between vehicle treated and in treated group. Only one patient showed side effects such as erythema and population.
Conclusion: Fumaria parviflora appears to be tolerated by most patients and the findings showed that its extract may be considered as an effective agent for treatment of chronic hand eczema.
Keywords: Eczema; Fumaria parviflora; Fumaric acid.
Conflict of interest statement
Figures
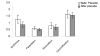
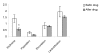
References
-
- Holden CA, Berth-Jones J. Eczema, Lichenification, Prurigo and Erythroderma. In: Burns T, Breathnach S, Cox N, Greffiths C, editors. Rook ’ s Text book of Dermatology. 8 th edition. Oxford: Wiley-Blackwell; 2010. pp. 876–934.
-
- James WD, Berger TG, Elson DM. Andrews’ Diseases of the skin clinical dermatology. Philadelphia: Saunders company; 2005. pp. 78–83.
-
- Avicenna . A1 Qanun Fil Tibb. New Delhi: Jamia Hamdard printing press; 1998. pp. 425–691.
-
- Mills S, Bone K. Principals and practice of phytotherapy modern herbal medicine. London: Churchill Livingstone; 2000. p. 254.
-
- Hagerman A, Harvey-Mueller I, Makkar HPS. Quantification of Tannins in Tree Foliage-A Laboratory Manual for FAO/IAEA. IAEA. 2000;1:21–4.
LinkOut - more resources
Full Text Sources
Other Literature Sources
