Alzheimer's disease multiple intervention trial (ADMIT): study protocol for a randomized controlled clinical trial
- PMID: 22737979
- PMCID: PMC3518146
- DOI: 10.1186/1745-6215-13-92
Alzheimer's disease multiple intervention trial (ADMIT): study protocol for a randomized controlled clinical trial
Abstract
Background: Given the current lack of disease-modifying therapies, it is important to explore new models of longitudinal care for older adults with dementia that focus on improving quality of life and delaying functional decline. In a previous clinical trial, we demonstrated that collaborative care for Alzheimer's disease reduces patients' neuropsychiatric symptoms as well as caregiver stress. However, these improvements in quality of life were not associated with delays in subjects' functional decline.
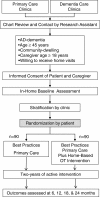
Trial design: Parallel randomized controlled clinical trial with 1:1 allocation.
Participants: A total of 180 community-dwelling patients aged ≥45 years who are diagnosed with possible or probable Alzheimer's disease; subjects must also have a caregiver willing to participate in the study and be willing to accept home visits. Subjects and their caregivers are enrolled from the primary care and geriatric medicine practices of an urban public health system serving Indianapolis, Indiana, USA.
Interventions: All patients receive best practices primary care including collaborative care by a dementia care manager over two years; this best practices primary care program represents the local adaptation and implementation of our prior collaborative care intervention in the urban public health system. Intervention patients also receive in-home occupational therapy delivered in twenty-four sessions over two years in addition to best practices primary care. The focus of the occupational therapy intervention is delaying functional decline and helping both subjects and caregivers adapt to functional impairments. The in-home sessions are tailored to the specific needs and goals of each patient-caregiver dyad; these needs are expected to change over the course of the study.
Objective: To determine whether best practices primary care plus home-based occupational therapy delays functional decline among patients with Alzheimer's disease compared to subjects treated in the control group.
Outcomes: The primary outcome is the Alzheimer's Disease Cooperative Studies Group Activities of Daily Living Scale; secondary outcome measures are two performance-based measures including the Short Physical Performance Battery and Short Portable Sarcopenia Measure. Outcome assessments for both the caregiver-reported scale and subjects' physical performance scales are completed in the subject's home.
Randomization: Eligible patient-care giver dyads will be stratified by clinic type and block randomized with a computer developed randomization scheme using a 1:1 allocation ratio.
Blinding: Single blinded. Research assistants completing the outcome assessments were blinded to the subjects' treatment group.
Trial status: Ongoing CLINICALTRIAL.GOV IDENTIFIER: NCT01314950; date of completed registration 10 March 2011; date first patient randomized 9 March 2011.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical