Distribution and processing of a disintegrin and metalloproteinase with thrombospondin motifs-4, aggrecan, versican, and hyaluronan in equine digital laminae
- PMID: 22738056
- PMCID: PMC3535468
- DOI: 10.2460/ajvr.73.7.1035
Distribution and processing of a disintegrin and metalloproteinase with thrombospondin motifs-4, aggrecan, versican, and hyaluronan in equine digital laminae
Abstract
Objective: To determine the expression and distribution of a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4), its substrates aggrecan and versican, and their binding partner hyaluronan in laminae of healthy horses.
Sample: Laminae from the forelimb hooves of 8 healthy horses.
Procedures: Real-time quantitative PCR assay was used for gene expression analysis. Hyaluronidase, chondroitinase, and keratanase digestion of lamina extracts combined with SDS-PAGE and western blotting were used for protein and proteoglycan analysis. Immunofluorescent and immunohistochemical staining of tissue sections were used for protein and hyaluronan localization.
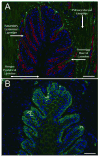
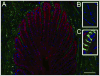
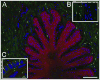
Results: Genes encoding ADAMTS-4, aggrecan, versican, and hyaluronan synthase II were expressed in laminae. The ADAMTS-4 was predominantly evident as a 51-kDa protein bearing a catalytic site neoepitope indicative of active enzyme and in situ activity, which was confirmed by the presence of aggrecan and versican fragments bearing ADAMTS-4 cleavage neoepitopes in laminar protein extracts. Aggrecan, versican, and hyaluronan were localized to basal epithelial cells within the secondary epidermal laminae. The ADAMTS-4 localized to these cells but was also present in some cells in the dermal laminae.
Conclusions and clinical relevance: Within digital laminae, versican exclusively and aggrecan primarily localized within basal epithelial cells and both were constitutively cleaved by ADAMTS-4, which therefore contributed to their turnover. On the basis of known properties of these proteoglycans, it is possible that they can protect the basal epithelial cells of horses from biomechanical and concussive stress.
Figures





References
-
- Pollitt CC. The Anatomy and Physiology of the Suspensory Apparatus of the Distal Phalanx. Veterinary Clinics of North America: Equine Practice. 2010;26:29–49. Advances in Laminitis, Part I. - PubMed
-
- French KR, Pollitt CC. Equine laminitis: loss of hemidesmosomes in hoof secondary epidermal lamellae correlates to dose in an oligofructose induction model: an ultrastructural study. Equine Vet J. 2004;36:230–235. - PubMed
-
- Black SJ. Extracellular matrix, leukocyte migration and laminitis. Vet Immunol Immunopathol. 2009;129:161–163. - PubMed
-
- Budras KD, Hullinger RL, Sack WO. Light and electron microscopy of keratinization in the laminar epidermis of the equine hoof with reference to laminitis. Am J Vet Res. 1989;50:1150–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

