Severe brief pressure-controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice
- PMID: 22738159
- PMCID: PMC3472682
- DOI: 10.1089/neu.2011.2303
Severe brief pressure-controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice
Abstract
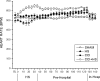
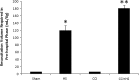
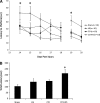
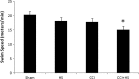
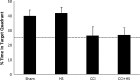
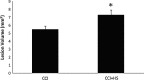
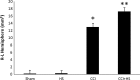
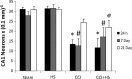
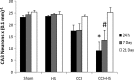
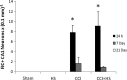
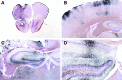
Hypotension after traumatic brain injury (TBI) worsens outcome. We published the first report of TBI plus hemorrhagic shock (HS) in mice using a volume-controlled approach and noted increased neuronal death. To rigorously control blood pressure during HS, a pressure-controlled HS model is required. Our hypothesis was that a brief, severe period of pressure-controlled HS after TBI in mice will exacerbate functional deficits and neuropathology versus TBI or HS alone. C57BL6 male mice were randomized into four groups (n=10/group): sham, HS, controlled cortical impact (CCI), and CCI+HS. We used a pressure-controlled shock phase (mean arterial pressure [MAP]=25-27 mm Hg for 35 min) and its treatment after mild to moderate CCI including, a 90 min pre-hospital phase, during which lactated Ringer's solution was given to maintain MAP >70 mm Hg, and a hospital phase, when the shed blood was re-infused. On days 14-20, the mice were evaluated in the Morris water maze (MWM, hidden platform paradigm). On day 21, the lesion and hemispheric volumes were quantified. Neuropathology and hippocampal neuron counts (hematoxylin and eosin [H&E], Fluoro-Jade B, and NeuN) were evaluated in the mice (n=60) at 24 h, 7 days, or 21 days (n=5/group/time point). HS reduced MAP during the shock phase in the HS and CCI+HS groups (p<0.05). Fluid requirements during the pre-hospital phase were greatest in the CCI+HS group (p<0.05), and were increased in HS versus sham and CCI animals (p<0.05). MWM latency was increased on days 14 and 15 after CCI+HS (p<0.05). Swim speed and visible platform latency were impaired in the CCI+HS group (p<0.05). CCI+HS animals had increased contusion volume versus the CCI group (p<0.05). Hemispheric volume loss was increased 33.3% in the CCI+HS versus CCI group (p<0.05). CA1 cell loss was seen in CCI+HS and CCI animals at 24 h and 7 days (p<0.05). CA3 cell loss was seen after CCI+HS (p<0.05 at 24 h and 7 days). CA1 cell loss at 21 days was seen only in CCI+HS animals (p<0.05). Brief, severe, pressure-controlled HS after CCI produces robust functional deficits and exacerbates neuropathology versus CCI or HS alone.
Figures








References
-
- Abrahamson E.E. Ikonomovic M.D. Dixon C.E. DeKosky S.T. Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels. Ann. Neurol. 2009;66:407–414. - PubMed
-
- Bulger E.M. May S. Brasel K.J. Schreiber M. Kerby J.D. Tisherman S.A. Newgard C. Slutsky A. Coimbra R. Emerson S. Minei J.P. Bardarson B. Kudenchuk P. Baker A. Christenson J. Idris A. Davis D. Fabian T.C. Aufderheide T.P. Callaway C. Williams C. Banek J. Vaillancourt C. van Heest R. Sopko G. Hata J.S. Hoyt D.B. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2010;304:1455–1464. ROC Investigators. - PMC - PubMed
-
- Carrillo P. Takasu A. Safar P. Tisherman S. Stezoski S.W. Stolz G. Dixon C.E. Radovsky A. Prolonged severe hemorrhagic shock and resuscitation in rats does not cause subtle brain damage. J. Trauma. 1998;45:239–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

