Topical application of ex vivo expanded endothelial progenitor cells promotes vascularisation and wound healing in diabetic mice
- PMID: 22738265
- PMCID: PMC7950455
- DOI: 10.1111/j.1742-481X.2012.01010.x
Topical application of ex vivo expanded endothelial progenitor cells promotes vascularisation and wound healing in diabetic mice
Abstract
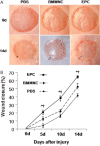
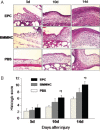
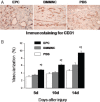
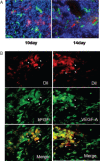
Impaired wound healing leading to skin ulceration is a serious complication of diabetes and may be caused by defective angiogenesis. Endothelial progenitor cells (EPCs) can augment neovascularisation in the ischaemic tissue. Experiments were performed to test the hypothesis that locally administered EPCs can promote wound healing in diabetes. Full-thickness skin wounds were created on the dorsum of diabetic mice. EPCs were obtained from bone marrow mononuclear cells (BMMNCs) and applied topically to the wound immediately after surgery. Vehicle and non-selective BMMNCs were used as controls. Wound size was measured on days 5, 10 and 14 after treatment, followed by resection, histological analysis and quantification of vascularity. Topical application of EPCs significantly promoted wound healing, as assessed by closure rate and wound vascularity. Immunostaining revealed that transplanted EPCs induced increased expression of vascular endothelial growth factor and basic fibroblast growth factor. Few EPCs were observed in the neovasculature based on in vivo staining of the functional vasculature. Ex vivo expanded EPCs promote wound healing in diabetic mice via mechanisms involving increased local cytokine expression and enhanced neovascularisation of the wound. This strategy exploiting the therapeutic capacity of autologously derived EPCs may be a novel approach to skin repair in diabetes.
Keywords: Basic fibroblast growth factor; Endothelial progenitor cells; Neovascularisation; Vascular endothelial growth factor; Wound healing.
© 2012 The Authors. International Wound Journal © 2012 John Wiley & Sons Ltd and Medicalhelplines.com Inc.
Figures
References
-
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361: 1545–51. - PubMed
-
- Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992;267:10931–4. - PubMed
-
- Risau W. Differentiation of endothelium. FASEB J 1995;9:926–33. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–7. - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

