Re-engineering aryl methylcarbamates to confer high selectivity for inhibition of Anopheles gambiae versus human acetylcholinesterase
- PMID: 22738634
- PMCID: PMC3389130
- DOI: 10.1016/j.bmcl.2012.05.103
Re-engineering aryl methylcarbamates to confer high selectivity for inhibition of Anopheles gambiae versus human acetylcholinesterase
Erratum in
- Bioorg Med Chem Lett. 2013 Oct 15;23(20):5752
Abstract
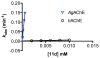
To identify potential human-safe insecticides against the malaria mosquito we undertook an investigation of the structure-activity relationship of aryl methylcarbamates inhibitors of acetylcholinesterase (AChE). Compounds bearing a β-branched 2-alkoxy or 2-thioalkyl group were found to possess good selectivity for inhibition of Anopheles gambiae AChE over human AChE; up to 530-fold selectivity was achieved with carbamate 11d. A 3D QSAR model is presented that is reasonably consistent with log inhibition selectivity of 34 carbamates. Toxicity of these compounds to live Anopheles gambiae was demonstrated using both tarsal contact (filter paper) and topical application protocols.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- World Malaria Report 2011. The World Health Organization; http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf.
-
- Garner P, Graves PM. Plos Medicine. 2005;2:287. - PMC - PubMed
- Burrows JN, Chibale K, Wells TNC. Curr Top Med Chem. 2011;11:1226. - PubMed
- Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GAT. Plos Medicine. 2005;2:338. - PMC - PubMed
- Wells TNC, Alonso PL, Gutteridge WE. Nature Rev Drug Disc. 2009;8:879. - PubMed
-
- Lindblade KA, Eisele TP, Gimnig JE, Alaii JA, Odhiambo F, ter Kuile FO, Hawley WA, Wannemuehler KA, Phillips-Howard PA, Rosen DH, Nahlen BL, Terlouw DJ, Adazu K, Vulule JM, Slutsker L. JAMA. 2004;291:2571. - PubMed
- Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Malaria J. 2010:9. - PMC - PubMed
- Rietveld A, Kurdova-Mintcheva R. Eliminating Malaria: Learning from the Past, Looking Ahead. World Health Organization; 2011.
- Lim SS, Fullman N, Stokes A, Ravishankar N, Masiye F, Murray CJL, Gakidou E. Plos Medicine. 2011:8. - PMC - PubMed
-
- Nauen R. Public Health - Bayer Environ Sci J. 2006;18:8.
-
- N’Guessan R, Boko P, Odjo A, Knols B, Akogbeto M, Rowland M. Trop Med Int Health. 2009;14:389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

