Dominant-negative effect of SCN5A N-terminal mutations through the interaction of Na(v)1.5 α-subunits
- PMID: 22739120
- PMCID: PMC3444234
- DOI: 10.1093/cvr/cvs211
Dominant-negative effect of SCN5A N-terminal mutations through the interaction of Na(v)1.5 α-subunits
Abstract
Aims: Brugada syndrome (BrS) is an autosomal-inherited cardiac arrhythmia characterized by an ST-segment elevation in the right precordial leads of the electrocardiogram and an increased risk of syncope and sudden death. SCN5A, encoding the cardiac sodium channel Na(v)1.5, is the main gene involved in BrS. Despite the fact that several mutations have been reported in the N-terminus of Na(v)1.5, the functional role of this region remains unknown. We aimed to characterize two BrS N-terminal mutations, R104W and R121W, a construct where this region was deleted, ΔNter, and a construct where only this region was present, Nter.
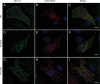
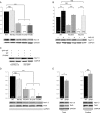
Methods and results: Patch-clamp recordings in HEK293 cells demonstrated that R104W, R121W, and ΔNter abolished the sodium current I(Na). Moreover, R104W and R121W mutations exerted a strong dominant-negative effect on wild-type (WT) channels. Immunocytochemistry of rat neonatal cardiomyocytes revealed that both mutants were mostly retained in the endoplasmic reticulum and that their co-expression with WT channels led to WT channel retention. Furthermore, co-immunoprecipitation experiments showed that Na(v)1.5-subunits were interacting with each other, even when mutated, deciphering the mutation dominant-negative effect. Both mutants were mostly degraded by the ubiquitin-proteasome system, while ΔNter was addressed to the membrane, and Nter expression induced a two-fold increase in I(Na). In addition, the co-expression of N-terminal mutants with the gating-defective but trafficking-competent R878C-Na(v)1.5 mutant gave rise to a small I(Na).
Conclusion: This study reports for the first time the critical role of the Na(v)1.5 N-terminal region in channel function and the dominant-negative effect of trafficking-defective channels occurring through α-subunit interaction.
Figures





Comment in
-
Unexpected dominance: Brugada syndrome SCN5A variants exert negative dominance via α-subunit interaction.Cardiovasc Res. 2012 Oct 1;96(1):1-3. doi: 10.1093/cvr/cvs264. Epub 2012 Aug 7. Cardiovasc Res. 2012. PMID: 22871588 No abstract available.
References
-
- Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference: endorsed by the heart rhythm society and the european heart rhythm association. Circulation. 2005;111:659–670. - PubMed
-
- Wilde AA, Brugada R. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circ Res. 2011;108:884–897. - PubMed
-
- Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. - PubMed
-
- Keller DI, Rougier JS, Kucera JP, Benammar N, Fressart V, Guicheney P, et al. Brugada syndrome and fever: Genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res. 2005;67:510–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

