IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection
- PMID: 22739232
- PMCID: PMC3835350
- DOI: 10.1038/mi.2012.49
IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection
Abstract
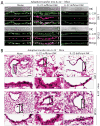
Influenza infection primarily targets the upper respiratory system, leading to a severe destruction of the epithelial cell layer. The role of immune cells in the regeneration of tracheal and bronchial epithelial cells is not well defined. Here, we investigated the production of pro-constructive cytokine, Interleukin-22 (IL-22), in the bronchoalveolar lavage (BAL), trachea, lung tissue, and spleen during influenza infection. We found that conventional natural killer (NK) cells (NCR1(+)NK1.1(+)CD127(-)RORγt(-)) were the predominant IL-22-producers in the BAL, trachea, and lung tissues. Tracheal epithelial cells constitutively expressed high levels of IL-22R and underwent active proliferation in response to IL-22 in the wild-type mice. Infection of IL-22(-/-) mice with influenza virus resulted in a severe impairment in the regeneration of tracheal epithelial cells. In addition, IL-22(-/-) mice continued to lose body weight even after 10 days post infection without any recovery. Tracheal epithelial cell proliferation was significantly reduced in IL-22(-/-) mice during influenza infection. Adoptive transfer of IL-22-sufficient but not IL-22-deficient NK cells into IL-22(-/-) mice restored the tracheal/bronchial epithelial cell regeneration and conferred protection against inflammation. Our findings strongly suggest that conventional NK cells have evolved to both kill virus-infected cells and also to provide vital cytokines for tissue regeneration.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

