Glucagon-like peptide-1 (GLP-1) protects vascular endothelial cells against advanced glycation end products (AGEs)-induced apoptosis
- PMID: 22739729
- PMCID: PMC3560786
- DOI: 10.12659/msm.883207
Glucagon-like peptide-1 (GLP-1) protects vascular endothelial cells against advanced glycation end products (AGEs)-induced apoptosis
Abstract
Background: The peptide glucagon-like peptide-1 (GLP-1) is a hormone secreted by intestinal L cells in response to food intake. GLP-1 has been proposed as the basis of emerging therapy for patients with type 2 diabetes. However, the effects of GLP-1 on vascular injury in diabetes have not been identified. Advanced glycation end products (AGEs) induce endothelial cell apoptosis and have been implicated in the process of vascular complications from diabetes.
Material/methods: The aim of this work was to investigate whether and how GLP-1 protects endothelial cells from apoptosis induced by AGEs. Human umbilical vein endothelial cells (HUVECs) were treated with AGEs (200 µg/mL) for 48 h in the presence or absence of GLP-1. Cell morphology, viability, apoptosis, ratio of Bcl-2 protein to Bax protein, cytochrome c release, and activity of caspase-9 and -3 were determined.
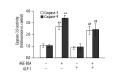
Results: Treatment of cells with AGEs led to cell morphology changes and decreased cell viability, resulting in apoptosis. GLP-1 alone increased cell viability in a concentration-dependent manner. GLP-1 partially inhibited AGEs-induced apoptosis in HUVECs. GLP-1 increased Bcl-2/Bax ratio, reduced cytochrome c levels in the cytoplasm, and reduced the activity of caspase-9 and -3 in AGEs-treated HUVECs.
Conclusions: AGEs induces apoptosis via the mitochondrion-cytochrome c-caspase protease pathway, and GLP-1 protects endothelial cells by interfering with this mechanism. GLP-1 may represent an anti-apoptotic agent in the treatment of vascular complications arising from diabetes.
Figures



References
-
- Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–99. - PubMed
-
- Meerwaldt R, van der Vaart MG, van Dam GM, et al. Clinical Relevance of Advanced Glycation Endproducts for Vascular Surgery. Eur J Vasc Endovasc. 2008;36:125–31. - PubMed
-
- Warboys CM, Toh HB, Fraser PA. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci. 2009;50:1319–28. - PubMed
-
- Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217–24. - PubMed
-
- Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

