Differential effects of peripheral versus central coadministration of QX-314 and capsaicin on neuropathic pain in rats
- PMID: 22739765
- PMCID: PMC3421838
- DOI: 10.1097/ALN.0b013e318260de41
Differential effects of peripheral versus central coadministration of QX-314 and capsaicin on neuropathic pain in rats
Abstract
Background: Neuropathic pain is common and difficult to treat. Recently a technique was developed to selectively inhibit nociceptive inputs by simultaneously applying two drugs: capsaicin, a transient receptor potential vanilloid receptor-1 channel activator, and QX-314, a lidocaine derivative that intracellularly blocks sodium channels. We used this technique to investigate whether transient receptor potential vanilloid receptor 1-expressing nociceptors contribute to neuropathic pain.
Methods: The rat chronic constriction injury model was used to induce neuropathic pain in order to test the analgesic effects of both peripheral (perisciatic) and central (intrathecal) administration of the QX-314/capsaicin combination. The Hargreaves and von Frey tests were used to monitor evoked pain-like behaviors and visual observations were used to rank spontaneous pain-like behaviors.
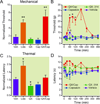
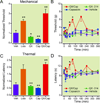
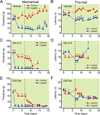
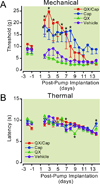
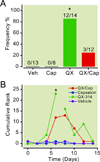
Results: Perisciatic injections of the QX-314/capsaicin combination transiently increased the withdrawal thresholds by approximately 3-fold, for mechanical and thermal stimuli in rats (n = 6/group) with nerve injuries suggesting that peripheral transient receptor potential vanilloid receptor 1-expressing nociceptors contribute to neuropathic pain. In contrast, intrathecal administration of the QX-314/capsaicin combination did not alleviate pain-like behaviors (n = 5/group). Surprisingly, intrathecal QX-314 alone (n = 9) or in combination with capsaicin (n = 8) evoked spontaneous pain-like behaviors.
Conclusions: Data from the perisciatic injections suggested that a component of neuropathic pain was mediated by peripheral nociceptive inputs. The role of central nociceptive terminals could not be determined because of the severe side effects of the intrathecal drug combination. We concluded that only peripheral blockade of transient receptor potential vanilloid receptor 1-expressing nociceptive afferents by the QX-314/capsaicin combination was effective at reducing neuropathic allodynia and hyperalgesia.
Figures


Similar articles
-
Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents.Anesthesiology. 2009 Jul;111(1):127-37. doi: 10.1097/ALN.0b013e3181a915e7. Anesthesiology. 2009. PMID: 19512868 Free PMC article.
-
Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation.PLoS One. 2012;7(9):e44023. doi: 10.1371/journal.pone.0044023. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22962595 Free PMC article.
-
Nociceptor-selective peripheral nerve block induces delayed mechanical hypersensitivity and neurotoxicity in rats.Anesthesiology. 2014 Apr;120(4):976-86. doi: 10.1097/ALN.0000000000000088. Anesthesiology. 2014. PMID: 24296762
-
Elucidation of pathophysiology and treatment of neuropathic pain.Cent Nerv Syst Agents Med Chem. 2012 Dec;12(4):304-14. doi: 10.2174/187152412803760645. Cent Nerv Syst Agents Med Chem. 2012. PMID: 23033930 Review.
-
Topical Capsaicin for the Treatment of Neuropathic Pain.Curr Drug Metab. 2021;22(3):198-207. doi: 10.2174/1389200221999201116143701. Curr Drug Metab. 2021. PMID: 33198614
Cited by
-
External QX-314 inhibits evoked cranial primary afferent synaptic transmission independent of TRPV1.J Neurophysiol. 2014 Dec 1;112(11):2697-706. doi: 10.1152/jn.00316.2014. Epub 2014 Sep 3. J Neurophysiol. 2014. PMID: 25185814 Free PMC article.
-
Optogenetic Activation of Non-Nociceptive Aβ Fibers Induces Neuropathic Pain-Like Sensory and Emotional Behaviors after Nerve Injury in Rats.eNeuro. 2018 Feb 15;5(1):ENEURO.0450-17.2018. doi: 10.1523/ENEURO.0450-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29468190 Free PMC article.
-
Quaternary Lidocaine Derivatives: Past, Present, and Future.Drug Des Devel Ther. 2021 Jan 14;15:195-207. doi: 10.2147/DDDT.S291229. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33469271 Free PMC article. Review.
-
Swim therapy reduces mechanical allodynia and thermal hyperalgesia induced by chronic constriction nerve injury in rats.Pain Med. 2013 Apr;14(4):516-25. doi: 10.1111/pme.12057. Epub 2013 Feb 25. Pain Med. 2013. PMID: 23438327 Free PMC article.
-
VGLUT2 controls heat and punctuate hyperalgesia associated with nerve injury via TRPV1-Cre primary afferents.PLoS One. 2015 Jan 23;10(1):e0116568. doi: 10.1371/journal.pone.0116568. eCollection 2015. PLoS One. 2015. PMID: 25615623 Free PMC article.
References
-
- Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 2009;10:918–929. - PubMed
-
- Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: Review and implications. Neurology. 2007;68:1178–1182. - PubMed
-
- O'Connor AB. Neuropathic pain: Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. - PubMed
-
- Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. - PubMed
-
- Attal N, Cruccu G, Haanpää M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

