Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress
- PMID: 22739983
- PMCID: PMC3388241
- DOI: 10.1038/cddis.2012.71
Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress
Abstract
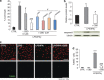
Cannabidiol (CBD) is the most abundant cannabinoid in Cannabis sativa that has no psychoactive properties. CBD has been approved to treat inflammation, pain and spasticity associated with multiple sclerosis (MS), of which demyelination and oligodendrocyte loss are hallmarks. Thus, we investigated the protective effects of CBD against the damage to oligodendrocyte progenitor cells (OPCs) mediated by the immune system. Doses of 1 μM CBD protect OPCs from oxidative stress by decreasing the production of reactive oxygen species. CBD also protects OPCs from apoptosis induced by LPS/IFNγ through the decrease of caspase 3 induction via mechanisms that do not involve CB1, CB2, TRPV1 or PPARγ receptors. Tunicamycin-induced OPC death was attenuated by CBD, suggesting a role of endoplasmic reticulum (ER) stress in the mode of action of CBD. This protection against ER stress-induced apoptosis was associated with reduced phosphorylation of eiF2α, one of the initiators of the ER stress pathway. Indeed, CBD diminished the phosphorylation of PKR and eiF2α induced by LPS/IFNγ. The pro-survival effects of CBD in OPCs were accompanied by decreases in the expression of ER apoptotic effectors (CHOP, Bax and caspase 12), and increased expression of the anti-apoptotic Bcl-2. These findings suggest that attenuation of the ER stress pathway is involved in the 'oligoprotective' effects of CBD during inflammation.
Figures




References
-
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. - PubMed
-
- Sastre-Garriga J, Vila C, Clissold S, Montalban X. THC and CBD oromucosal spray (Sativex(R)) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother. 11:627–637. - PubMed
-
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

