Suppression of PP2A is critical for protection of melanoma cells upon endoplasmic reticulum stress
- PMID: 22739989
- PMCID: PMC3388246
- DOI: 10.1038/cddis.2012.79
Suppression of PP2A is critical for protection of melanoma cells upon endoplasmic reticulum stress
Abstract
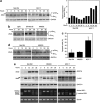
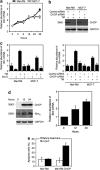
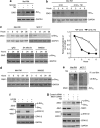
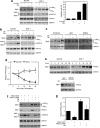
Endoplasmic reticulum (ER) stress triggers apoptosis by activating Bim in diverse types of cells, which involves dephosphorylation of Bim(EL) by protein phosphatase 2A (PP2A). However, melanoma cells are largely resistant to ER stress-induced apoptosis, suggesting that Bim activation is suppressed in melanoma cells undergoing ER stress. We show here that ER stress reduces PP2A activity leading to increased ERK activation and subsequent phosphorylation and proteasomal degradation of Bim(EL). Despite sustained upregulation of Bim at the transcriptional level, the Bim(EL) protein expression was downregulated after an initial increase in melanoma cells subjected to pharmacological ER stress. This was mediated by increased activity of ERK, whereas the phosphatase activity of PP2A was reduced by ER stress in melanoma cells. The increase in ERK activation was, at least in part, due to reduced dephosphorylation by PP2A, which was associated with downregulation of the PP2A catalytic C subunit. Notably, instead of direct dephosphorylation of Bim(EL), PP2A inhibited its phosphorylation indirectly through dephosphorylation of ERK in melanoma cells. Taken together, these results identify downregualtion of PP2A activity as an important protective mechanism of melanoma cells against ER stress-induced apoptosis.
Figures


References
-
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. - PubMed
-
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. - PubMed
-
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

