"Late" macroendosomes and acidic endosomes in vertebrate motor nerve terminals
- PMID: 22740045
- PMCID: PMC4209591
- DOI: 10.1002/cne.23176
"Late" macroendosomes and acidic endosomes in vertebrate motor nerve terminals
Abstract
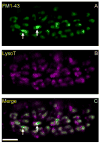
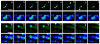
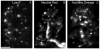
Activity at the vertebrate nerve-muscle synapse creates large macroendosomes (MEs) via bulk membrane infolding. Visualized with the endocytic probe FM1-43, most (94%) of the ∼25 MEs/terminal created by brief (30-Hz, 18-second) stimulation dissipate rapidly (∼1 minute) into vesicles. Others, however, remain for hours. Here we study these "late" MEs by using 4D live imaging over a period of ∼1 hour after stimulation. We find that some (51/398 or 13%) disappear spontaneously via exocytosis, releasing their contents into the extracellular milieu. Others (at least 15/1,960 or 1%) fuse or closely associate with a second class of endosomes that take up acidophilic dyes (acidic endosomes [AEs]). AEs are plentiful (∼47/terminal) and exist independent of stimulation. Unlike MEs, which exhibit Brownian motion, AEs exhibit directed motion (average, 83 nm/sec) on microtubules within and among terminal boutons. AEs populate the axon as well, where movement is predominantly retrograde. They share biochemical and immunohistochemical markers (e.g., lysosomal-associated membrane protein [LAMP-1]) with lysosomes. Fusion/association of MEs with AEs suggests a sorting/degradation pathway in nerve terminals wherein the role of AEs is similar to that of lysosomes. Based on our data, we propose that MEs serve as sorting endosomes. Thus their contents, which include plasma membrane proteins, vesicle proteins, and extracellular levels of Ca(2+) , can be targeted either toward the reformation and budding of synaptic vesicles, toward secretion via exocytosis, or toward a degradation process that utilizes AEs either for lysis within the terminal or for transport toward the cell body.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.
Figures

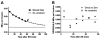








Similar articles
-
Macroendocytosis and endosome processing in snake motor boutons.J Physiol. 2007 Jul 1;582(Pt 1):243-62. doi: 10.1113/jphysiol.2007.130989. Epub 2007 May 3. J Physiol. 2007. PMID: 17478535 Free PMC article.
-
Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells.J Cell Biol. 1997 Apr 7;137(1):93-104. doi: 10.1083/jcb.137.1.93. J Cell Biol. 1997. PMID: 9105039 Free PMC article.
-
Syntaxin 7 mediates endocytic trafficking to late endosomes.J Biol Chem. 2000 Mar 3;275(9):6523-9. doi: 10.1074/jbc.275.9.6523. J Biol Chem. 2000. PMID: 10692457
-
Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction.Neuroscientist. 2005 Apr;11(2):138-47. doi: 10.1177/1073858404271679. Neuroscientist. 2005. PMID: 15746382 Review.
-
Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular.Int J Mol Sci. 2020 Apr 8;21(7):2576. doi: 10.3390/ijms21072576. Int J Mol Sci. 2020. PMID: 32276321 Free PMC article. Review.
Cited by
-
Regulatory role of sorting nexin 5 in protein stability and vesicular targeting of vesicular acetylcholine transporter to synaptic vesicle-like vesicles in PC12 cells.J Biomed Res. 2020 Aug 21;35(5):339-350. doi: 10.7555/JBR.34.20200095. J Biomed Res. 2020. PMID: 34230437 Free PMC article.
-
The synaptic maintenance problem: membrane recycling, Ca2+ homeostasis and late onset degeneration.Mol Neurodegener. 2013 Jul 8;8:23. doi: 10.1186/1750-1326-8-23. Mol Neurodegener. 2013. PMID: 23829673 Free PMC article. Review.
-
Visualization of endosome dynamics in living nerve terminals with four-dimensional fluorescence imaging.J Vis Exp. 2014 Apr 16;(86):51477. doi: 10.3791/51477. J Vis Exp. 2014. PMID: 24799002 Free PMC article.
References
-
- Agulhon C, Rostaing P, Ravassard P, Sagne C, Triller A, Giros B. Lysosomal amino acid transporter LYAAT-1 in the rat central nervous system: an in situ hybridization and immunohistochemical study. J Comp Neurol. 2003;462:71–89. - PubMed
-
- Andrejewski N, Punnonen E-L, Guhde G, Tanaka Y, Lullmann-Rauchl R, Hartmann D, von Figura K, Saftig P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem. 1999;274:12692–12701. - PubMed
-
- Banks P, Mayor D, Owen T. Effects of low temperatures on microtubules in the non-myelinated axons of post-ganglionic sympathetic nerves. Brain Res. 1975;83:277–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

